Submitted by Penny Peck on Wed, 09/09/2020 - 00:00
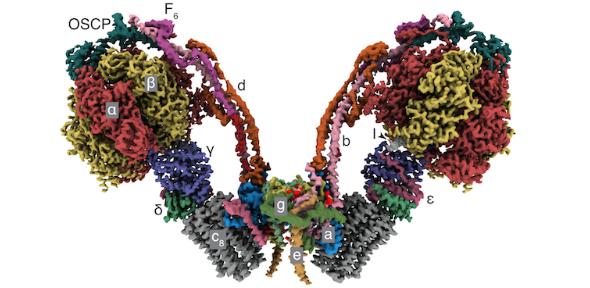
Tobias Spikes, Martin Montgomery and John Walker have solved the atomic resolution structure of dimeric ATP synthase from bovine mitochondria by cryo-electron microscopy.
The ATP synthases are complex molecular machines embedded in the inner membranes of mitochondria where they produce almost all the ATP required to sustain life by a mechanical rotary action. Single ATP synthases associate into dimers and form long rows, influencing the formation of characteristic cristae which change shape constantly.
The new work shows how the single ATP synthases in the dimers pivot in the membrane about wedge domains and translate relative to each other. It also shows how the torque to turn the enzyme’s rotor is generated by energy derived from the oxidation of sugars and fats in the food we eat, putting in place a crucial missing piece in understanding how this machine works.
The current structure is the culmination of a quest that John began more than 40 years ago to understand how ATP is made. It has been published in Proceedings of the National Academy of Science, USA.
John said: “I am very happy finally to have reached this pinnacle. I am extremely fortunate that many brilliant and talented students and colleagues have joined me in this long journey. I thank every one of them for their contributions.”
Congratulations to everyone involved!
Publication reference:
Structure of the dimeric ATP synthase from bovine mitochondria
Tobias E. Spikes, Martin G. Montgomery, John E. Walker
Proceedings of the National Academy of Sciences Sep 2020, 202013998; DOI: 10.1073/pnas.2013998117

