Submitted by Penny Peck on Tue, 23/01/2024 - 16:26
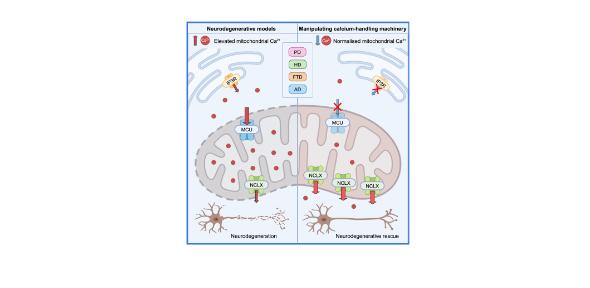
Mitochondrial dysfunction and calcium dysregulation are commonly observed in multiple neurodegenerative diseases, but mechanisms linking these are poorly understood in the context of neurodegeneration. In a study led by Dr Alex Whitworth (MRC Mitochondrial Biology Unit) and recently published in Cell Reports, Twyning et al investigate the intricate dynamics of mitochondrial calcium and its significance to neurodegeneration in animal models of Parkinson’s disease, Alzheimer’s disease, Frontotemporal Dementia and Huntington’s disease.
Using a single in vivo platform, Twyning et al reveal that in multiple models of neurodegeneration, neuronal mitochondria exhibit elevated basal calcium levels and reduced calcium buffering capacity. Physiological mitochondrial calcium homeostasis is maintained through the tightly regulated balance of calcium uptake via the mitochondrial calcium uniporter (MCU) complex and efflux via the Na+/Ca2+ transporter NCLX. The researchers went on to show that knocking down MCU or overexpressing NCLX was sufficient to normalise the pathologically elevated basal calcium levels. Strikingly, these interventions that modulated mitochondrial calcium flux were able to rescue neurodegenerative phenotypes, suppressing locomotion and longevity deficits in addition to neuronal loss, across a diverse spectrum of neurodegenerative models.
These findings reinforce mitochondrial dysfunction as a key contributor to neurodegenerative diseases of diverse origins, even in cases where protein aggregation is the dominant pathology. Twyning et al identify mitochondrial calcium homeostasis as a common mechanistic target for intervention. Importantly, the mitochondrial calcium flux machinery are druggable proteins making this mechanism attractive as a therapeutic target.
Reference
Twyning MJ, Tufi R, Gleeson TP, Kolodziej KM, Campesan S, Terriente-Felix A, Collins L, De Lazzari F, Giorgini F, Whitworth AJ.
Partial loss of MCU mitigates pathology in vivo across a diverse range of neurodegenerative disease models.
Cell Rep. 2024 Jan 17;43(2):113681. doi: 10.1016/j.celrep.2024.113681. Online ahead of print.PMID: 38236772

