ROLE OF CARDIOLIPIN IN MITOCHONDRIAL CARRIERS
All mitochondrial carriers are likely to have three tightly bound cardiolipin molecules, which are important for their structure and mechanism.
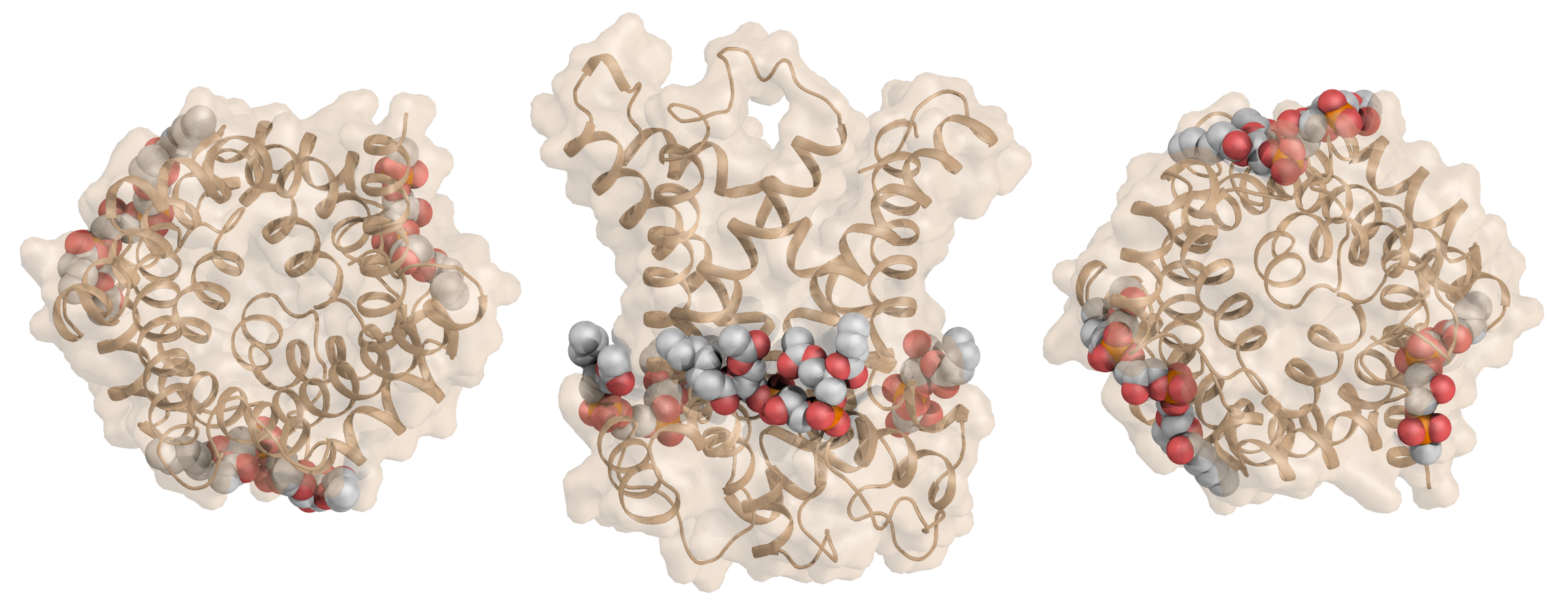
Figure Three cardiolipin molecules, shown in sphere representation, are tightly bound to the mitochondrial ADP/ATP carrier.
The cardiolipins are positioned with their phosphate groups at the N-terminal ends of the even-numbered and matrix α-helices [1] [2]. The phosphate moieities of cardiolipin provide hydrogen-bonding partners to the amide groups at the N-terminal ends of the helices, which are formed by glycine residues of the [YWF][KR]G and [YF]xG motifs [2]. Leaving unsatisfied hydrogen bond donors in a hydrophobic environment would be energetically unfavourable, and thus the cardiolipins act as a cap. The position of the cardiolipin phosphate groups also allows a favourable interaction with the positively charged side of the helix dipoles, stabilizing binding of cardiolipin, which is likely to carry two negative charges at physiological pH [2]. Recently we have shown that uncoupling proteins, which are of the same protein family, also have tightly bound cardiolipins [3].
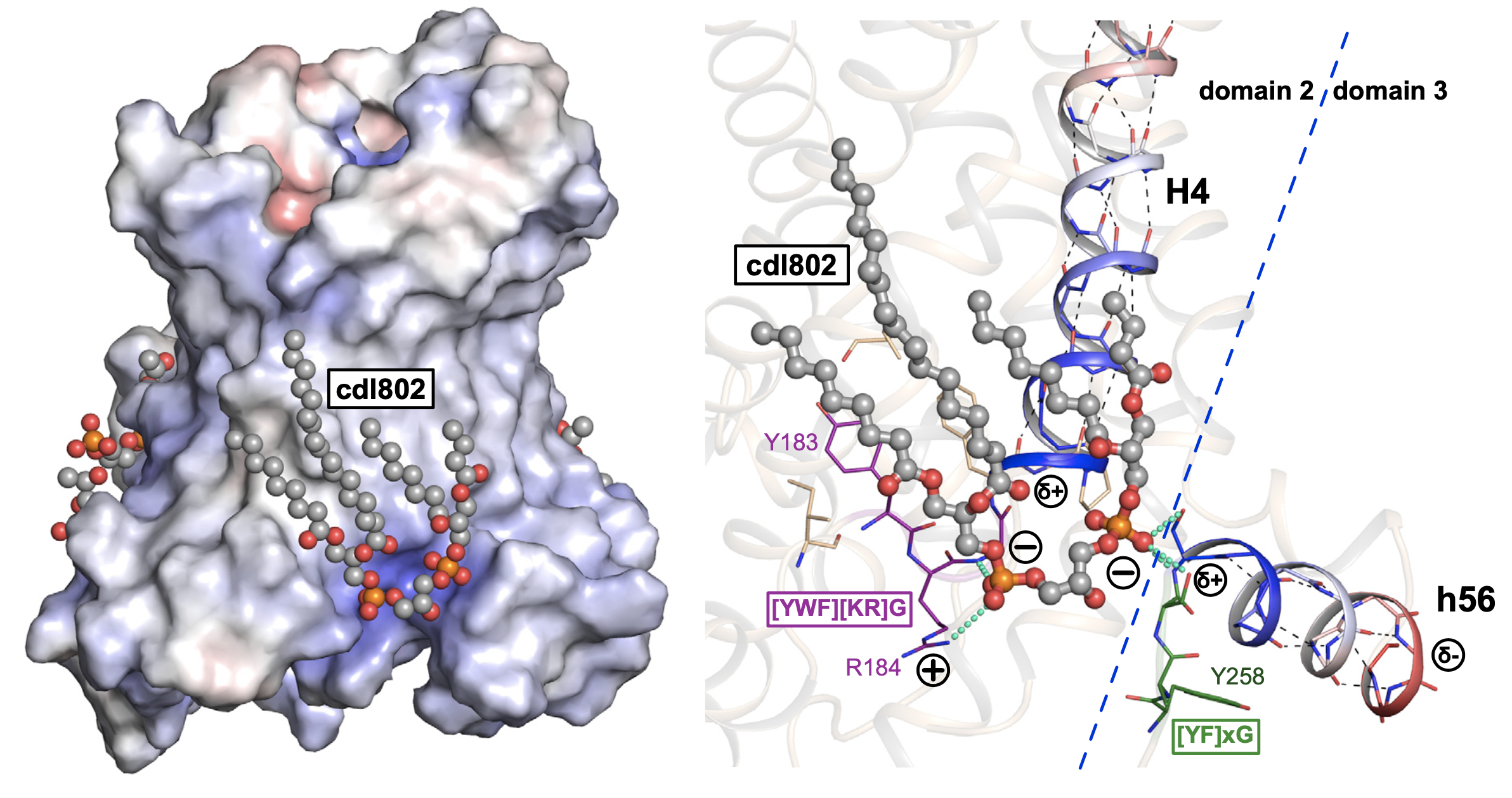
Figure Bound cardiolipin acts as an inter-domain bridge
Cardiolipin binding sites (ball-and-stick representation, with gray carbon atoms) with cdl802 highlighted. and detailed view of the binding site for cdl802. Cardiolipin phosphate groups form hydrogen bonds with the amide groups at the N-terminal ends of the even-numbered and matrix helices, and interact with the positively-charged ends of the helix dipoles. Residues in the conserved [YWF][RK]G and [YF]xG motifs are shown in purple and green, respectively. The helix dipoles associated with the even-numbered and matrix helices are shown (blue, d+, to red, d-).
We are interested in understanding the role of cardiolipin in the transport mechanism of mitochondrial carriers.
REFERENCES
- Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJ-M & Brandolin G (2003)
Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside.
Nature 426, 39-44 - Ruprecht JJ, Hellawell AM, Harding M, Crichton PG, McCoy AJ & Kunji ERS (2014)
Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism.
Proc Natl Acad Sci U S A 111, E426-34 - Lee Y, Willers C, Kunji ERS & Crichton PG (2015)
Uncoupling protein 1 binds one nucleotide per monomer and is stabilized by tightly bound cardiolipin.
Proc Natl Acad Sci U S A 112, 6973-8