Transcription factor-driven metabolic reprogramming
NRF2 and ATF4-mediated regulation of metabolic rewiring
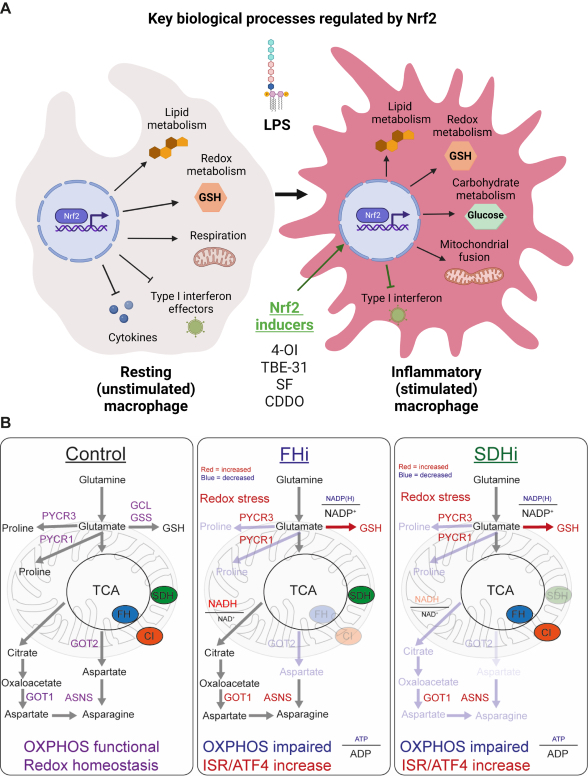
Transcription factor (TF)-mediated nuclear gene expression plays crucial roles in governing both physiological adaptations to cellular stress and the innate immune response. Our previous work has shown that activation of the NRF2 antioxidant response can suppress pro-inflammatory cytokine and type I IFN production [1,2]. Using unbiased proteomic and metabolomic profiling, respirometry, and confocal microscopy we demonstrated that NRF2 activation remodels macrophage intermediary metabolism and promotes mitochondrial fusion (Fig. 1A), with potential importance in its anti-inflammatory mechanism of action [2]. We’ve also demonstrated that NRF2 activation downstream of FH inhibition in inflammatory macrophages drives the release of inflammation-associated hormone GDF15, which is known to regulate systemic metabolism and tolerance to infection. In addition, using a multi-modal approach coupled with stable isotope-assisted nutrient tracing, we identified a crucial role for the integrated stress response (ISR) and ATF4 translation in counteracting redox stress and impaired amino acid biosynthesis downstream of TCA cycle inhibition and mitochondrial respiratory impairment in kidney epithelial cells (Fig. 1B) [3]. This may have potential ramifications for hereditary kidney cancer syndromes driven by loss of the tumour suppressors FH and SDH. Critical TFs downstream of innate immune signalling include NF-kB, AP-1, and the Interferon regulatory factors (IRFs), which drive the transcriptional regulation of pro-inflammatory cytokines, chemokines, and interferons. However, their precise role in regulating mitochondrial metabolic remodelling and their interaction with stress-induced TFs remains poorly defined and is an area of significant interest for the Ryan group.
References
- Mills E*, Ryan DG*, Prag H et al. (2018)
Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1.
Nature 556, 113-117. doi:10.1038/nature25986
*Joint-first - Ryan DG*, Knatko E*, Casey A et al.(2022)
Nrf2 activation reprograms intermediary metabolism and suppresses the type I interferon response.
iScience 25, 103827. doi:10.1016/j.isci.2022.103827
*Joint-first - Ryan DG, Yang M, Prag H et al.(2021)
Disruption of the TCA cycle reveals an ATF4-dependent integration of redox and amino acid metabolism.
eLife 10:e72593. doi:10.7554/eLife.72593