COMPLEX I MECHANISM
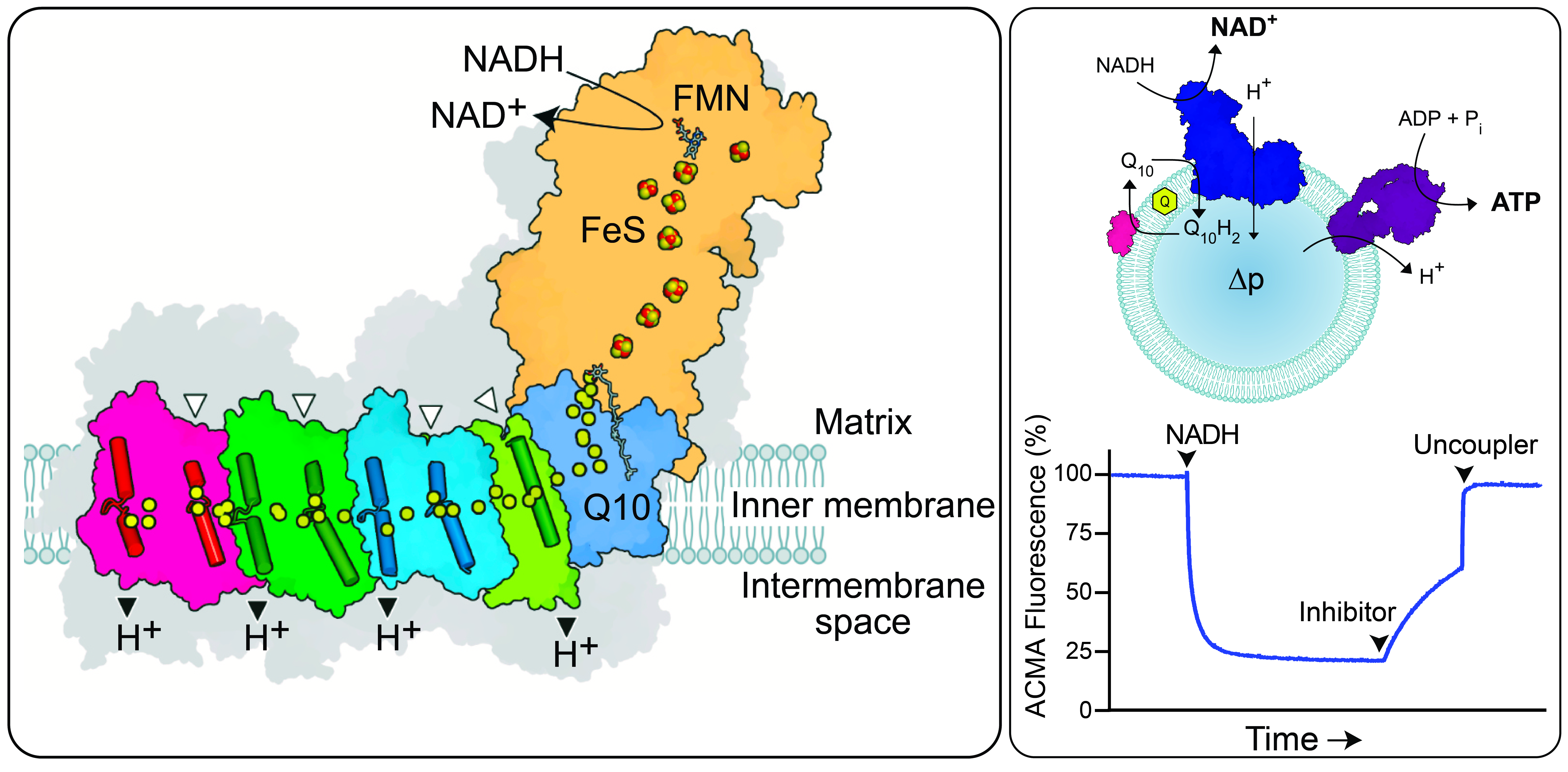
Figure Left: Schematic diagram showing the elements involved in enzymatic catalysis. Right: Schematic picture of a co-reconstituted proteoliposome system containing complex I, complex V (ATP synthase) and AOX (an ‘alternative’ quinol oxidase), and a fluorescence quenching experiment showing proton pumping.
During normal catalysis in the cell (forward electron transfer), complex I performs a redox reaction which passes electrons from the NADH produced by the tricarboxylic acid cycle along a chain of iron-sulphur (FeS) clusters to reduce ubiquinone to ubiquinol. The energy from the redox reaction is used to pump four protons [1] out of the mitochondrial matrix (or the cytoplasm in bacteria), contributing to the proton-motive force (PMF) that is used to generate ATP by the rotary catalysis of F1Fo ATP synthase (complex V). However, complex I catalysis is also reversible, and given sufficient PMF (such as produced by ATP hydrolysis by complex V operating in reverse), and a reduced quinol pool, electrons can be driven back through the enzyme to reduce NAD+ to NADH. This process is called reverse electron transfer (RET); it can occur under the physiological state of ischemia-reperfusion [2], and is also associated with substantial oxidative stress, due to reaction of the reduced flavin (in the NADH binding site) with O2 [3], [4].
While much has been learned over the years, important aspects of catalysis that are central to complex I function and dysfunction in vivo remain poorly understood, including the routes and mechanisms of proton transfer events, the role of ubisemiquinone, and the mechanism of precisely how electron transfer is coupled to proton pumping.
Our research tackles the mechanism of complex I catalysis by developing and combining a suite of biochemical and biophysical methods and toolkits:
- A bacterial model system for mutagenesis [5] that provides access to every core subunit in the enzyme, including the hydrophobic subunits encoded by the mitochondrial genome in mammalian species;
- Coupled native membrane vesicles and [1], [4], [6] co-reconstituted proteoliposomes containing complex I, complex V, quinone-cycling enzymes and native ubiquinone-10, to allow proton-pumping and coupled reactions to be assessed [1], [7], [8];
- Kinetics assays [9], [10] and biophysical techniques such as electron paramagnetic resonance spectroscopy [11], [12] to assess redox intermediates and semiquinone radicals.
References
- Jones AJY, Blaza JN, Varghese F & Hirst J (2017)
Respiratory complex I in Bos taurus and Paracoccus denitrificans pumps four protons across the membrane for every NADH oxidized.
J Biol Chem 292, 4987-4995 - Chouchani ET et al (2014)
Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS.
Nature 515, 431-435 - Kussmaul L & Hirst J (2006)
The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria.
Proc Natl Acad Sci USA 103, 7607-7612 - Pryde KR & Hirst J (2011)
Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer.
J Biol Chem 286, 18056-18065 - Jarman OD, Biner O, Wright JJ & Hirst J (2021)
Paracoccus denitrificans: a genetically tractable model system for studying respiratory complex I.
Sci Rep 11, 10143 - Fedor JG & Hirst J (2018)
Mitochondrial supercomplexes do not enhance catalysis by quinone channeling.
Cell Metab 28, 525-531 - Jones AJY, Blaza, JN, Bridges, HR, May, B, Moore, AL & Hirst J (2016)
A self-assembled respiratory chain that catalyzes NADH oxidation by ubiquinone-10 cycling between complex I and the alternative oxidase.
Angew Chem Int Ed Engl 55, 728-731 - Biner O, Fedor JG, Yin Z & Hirst J (2020)
Bottom-up construction of a minimal system for cellular respiration and energy regeneration.
ACS Synth Biol 9, 145-1459 - Blaza JN, Serreli R, Jones AJY, Mohammed K & Hirst J (2014)
Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes.
Proc Natl Acad Sci USA 111, 15735-15740 - Fedor JG, Jones, AJY, Di Luca A, Kaila VRI & Hirst J (2017)
Correlating kinetic and structural data on ubiquinone binding and reduction by respiratory complex I.
Proc Natl Acad Sci USA 114, 12737-12742 - Roessler MM, King MS, Robinson AJ, Armstrong FA, Harmer J & Hirst J (2010)
Direct assignment of EPR spectra to structurally defined iron-sulfur clusters in complex I by double electron–electron resonance.
Proc Natl Acad Sci USA 107, 1930-1935 - Wright JJ, Fedor JG, Hirst J & Roessler MM (2020)
Using a chimeric respiratory chain and EPR spectroscopy to determine the origin of semiquinone species previously assigned to mitochondrial complex I.
BMC Biol 18, 54