THE BIOGENESIS AND ASSEMBLY OF THE HUMAN ATP SYNTHASE
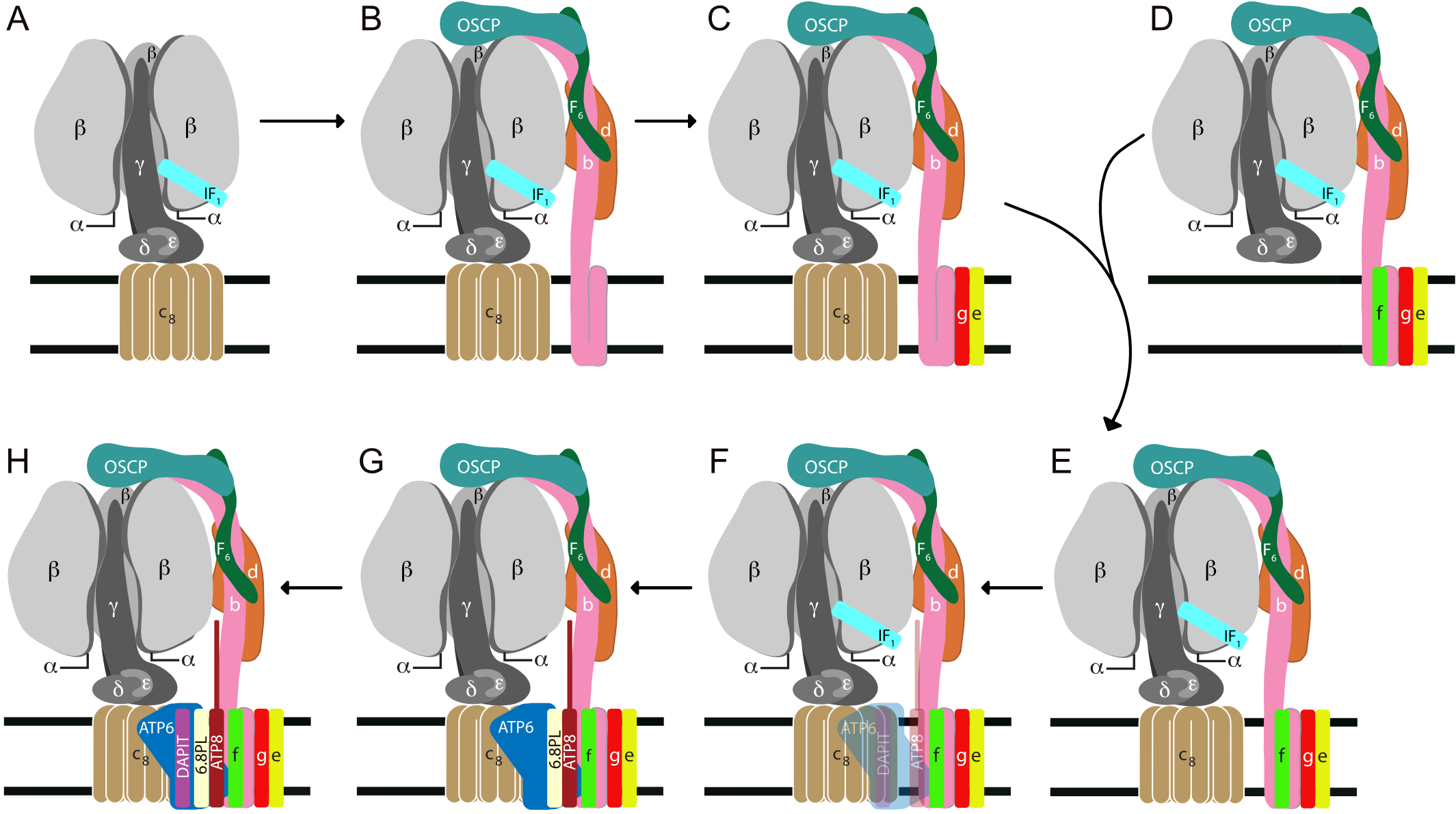
The ATP synthase in human mitochondria is a membrane bound assembly of 29 proteins of 18 kinds [1]. All but two membrane components are encoded in nuclear genes, synthesized on cytoplasmic ribosomes and imported into the matrix of the organelle. Here, they are assembled into the complex with ATP6 and ATP8, the products of overlapping genes in mitochondrial DNA [2]. By CRISPR-Cas9, we have disrupted individual human genes for the nuclear encoded subunits in the membrane portion of the enzyme. In each case, this leads to the formation of an intermediate vestigial ATPase complex. We have characterized these complexes [3][4]. Another vestigial ATPase has been characterized from ρ0-cells, which are devoid of subunits ATP6 and ATP8 [3]. These vestigial complexes provide a description of the pathway of assembly of the membrane domain (Fig. – Assembly summary). The key intermediate complex in this pathway, E, consists of the F1-c8 complex inhibited by the ATPase inhibitor protein, IF1, and attached to the peripheral stalk, with subunits e, f and g associated with the membrane domain of the peripheral stalk. This intermediate provides the template for insertion of ATP6 and ATP8 which are synthesized on mitochondrial ribosomes. The association of ATP6 and ATP8 with the complex is stabilized by the addition of the 6.8 proteolipid, and the complex is coupled to ATP synthesis at this point. A structure of the dimeric yeast Fo membrane domain [5] is consistent with this model of assembly.
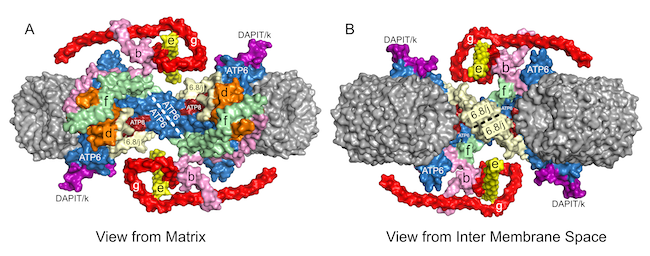
The human 6.8 proteolipid (yeast j-subunit) locks ATP6 and ATP8 into the membrane assembly, and the monomeric complexes dimerize via interactions between ATP6 subunits and between 6.8 proteolipids (j-subunits). The dimers are linked together back-to-face via dimer-dimer interactions involving subunit DAPIT (diabetes associated protein in insulin sensitive tissue; yeast subunit k), forming long oligomers along the edges of the cristae.
The remaining aims are as follows:
• to define the role of TMEM70 in the assembly of the membrane domain of the enzyme. Human mutations in this protein affect assembly of ATP synthase [6], and it has been suggested that it helps in the formation of the c8-rotor ring [7].
• to identify any other exogenous proteins involved in the process of assembly
• to establish the pathway of assembly of the peripheral stalk
• to determine whether the tri-methylation of Lys-43 in the c-subunit is part of the assembly pathway of ATP synthase
REFERENCES
- Citekey 10.1039/9781788010405 not found
- Fearnley IM & Walker JE (1986)
Two overlapping genes in bovine mitochondrial DNA encode membrane components of ATP synthase.
EMBO J 5, 2003-2008 - He J, Ford HC, Carroll J, Ding S, Fearnley IM & Walker JE (2017)
Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase.
Proc Natl Acad Sci U S A 114, 3409-3414 - He J, Carroll J, Ding S, Fearnley IM & Walker JE (2017)
Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase.
Proc Natl Acad Sci U S A 114, 9086-9091 - Guo H, Bueler SA & Rubinstein JL (2017)
Atomic model for the dimeric F region of mitochondrial ATP synthase.
Science 358, 936-940 - Cízková A, Stránecký V, Mayr JA, Tesarova M, Havlícková V, Paul J, Ivánek R, Kuss AW, Hansikova H, Kaplanová V, Vrbacký M, Hartmannová H, Nosková L, Honzík T, Drahota Z, Magner M, Hejzlarová K, Sperl W, Zeman J, Houštěk J & Kmoch S (2008)
TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy.
Nat Genet 40, 1288-1290 - Vrbacký M, Kovalčíková J, Chawengsaksophak K, Beck IM, Mráček T, Nůsková H, Sedmera D, Papoušek F, Kolář F, Sobol M, Hozák P, Sedlacek R & Houštěk J (2016)
Knockout of Tmem70 alters biogenesis of ATP synthase and leads to embryonal lethality in mice.
Hum Mol Genet 25, 4674-4685