MITOCHONDRIAL THERAPIES
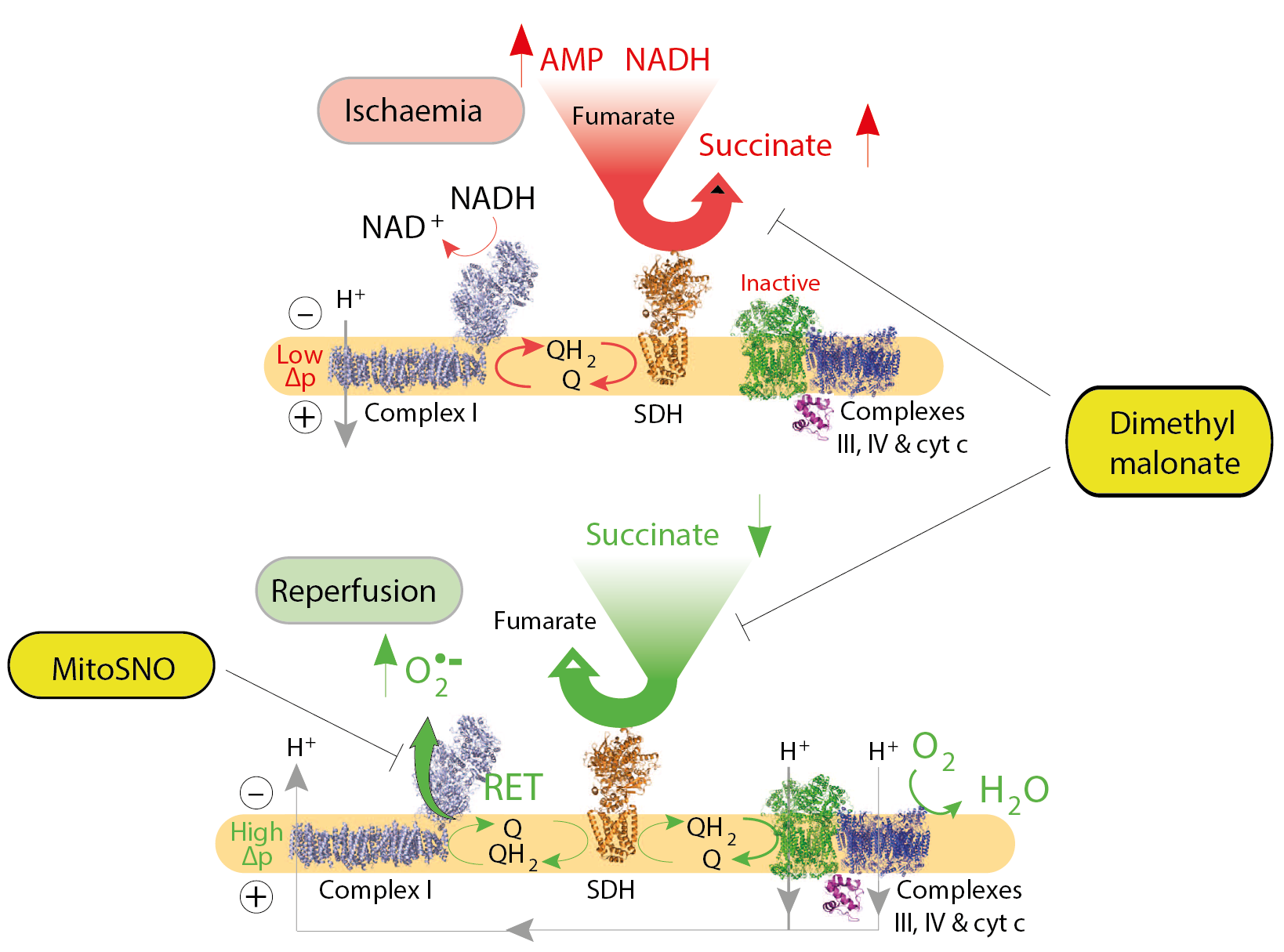
Ischaemia-reperfusion injury occurs when the blood supply to an organ is disrupted and then restored, and underlies many disorders, notably heart attack and stroke. While reperfusion of ischaemic tissue is essential for survival, it also initiates oxidative damage, cell death and aberrant immune responses through the generation of mitochondrial reactive oxygen species (ROS). The selective accumulation of the citric acid cycle intermediate succinate is a universal metabolic signature of ischaemia in a range of tissues and is responsible for mitochondrial ROS production by reverse electron transport (RET) through Complex I during reperfusion. Ischaemic succinate accumulation arises from reversal of succinate dehydrogenase, which in turn is driven by fumarate overflow from purine nucleotide breakdown and partial reversal of the malate/aspartate shuttle. After reperfusion, the accumulated succinate is rapidly re-oxidized by succinate dehydrogenase, driving extensive ROS generation by RET at mitochondrial complex I. Decreasing ischaemic succinate accumulation and/or succinate oxidation with dimethylmalonate is sufficient to ameliorate in vivo ischaemia-reperfusion injury in murine models of heart attack and stroke [1]. We can also prevent RET during reperfusion by reversibly inhibiting a specific cysteine residue on Complex I with a mitochondria-targeted nitrosylating reagent (MitoSNO). This has been shown to be beneficial when administered at reperfusion during in vivo ischaemia-reperfusion injury in murine models of heart attack [2].
As mitochondrial ROS contribute to a wider range of human diseases than ischaemia-reperfusion injury, antioxidants accumulated by mitochondria in vivo have been designed to limit oxidative damage. The most extensively studied of these mitochondria-targeted antioxidants is MitoQ, which contains the antioxidant quinone moiety covalently attached to a lipophilic triphenylphosphonium cation. MitoQ passes easily through all biological membranes and, because of its positive charge, is accumulated several hundred-fold within mitochondria driven by the mitochondrial membrane potential. MitoQ protects mitochondria against oxidative damage in vitro and following oral delivery and has now been used in a range of in vivo studies in rats and mice and in two phase II human trials. MitoQ is currently being tested by the National Institute of Aging for its effectiveness in extending lifespan.
REFERENCES
- (2014)
Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS.
Nature 515, 431-435 - (2013)
Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I.
Nat Med 19, 753-9