STRUCTURES OF MITOCHONDRIAL TRANSPORT PROTEINS
We have solved the atomic structures of the mitochondrial ADP/ATP carrier locked in the cytoplasmic state by carboxyatractyloside and in the matrix state by bongkrekic acid. Comparison of the two states explains the function of highly conserved sequence features and reveals that the transport mechanism is unique, involving the coordinated movement of six dynamic elements around a central translocation pathway.
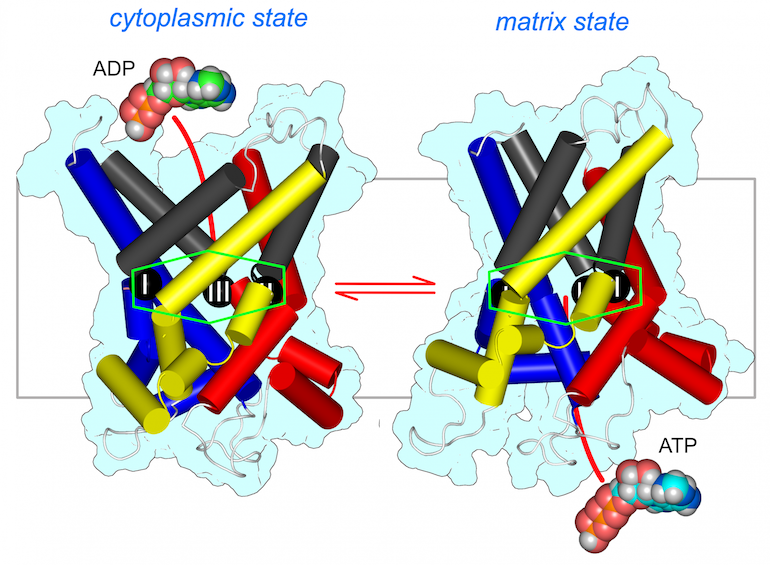
Figure Structural models of the mitochondrial ADP/ATP carrier in the cytoplasmic state (left) and the matrix state (right).
The structures provided important information in support of an alternating-access mechanism [1] [2]. The carrier has a single substrate binding site (green hexagon), which has three contact points (black spheres with Roman numerals) and is accessible from the cytoplasm and mitochondrial matrix, respectively. Conformational changes between the cytoplasmic and matrix state, induced by substrate-binding, involve outward rotations of the core elements of each domain (shown in primary colours), opening the matrix side, and inward rotation of the gate elements (shown in grey), closing the cytoplasmic side. The transition from the matrix to the cytoplasmic state involves the same elements operating in reverse.
Movie Morph between the two states, showing alternating acess, viewed from the cytoplasmic side (left) and matrix side (right) of the membrane. The gate elements (G1, G2, G3) are shown in grey, whereas the core elements (C1, C2 and C3) are shown in primary colours. If the movie does not show, refresh the page.
We are interested in using x-ray crystallography to solve the atomic structures of mitochondrial transport proteins in different states.
REFERENCES
- Ruprecht JJ, Hellawell AM, Harding M, Crichton PG, McCoy AJ & Kunji ERS (2014)
Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism.
Proc Natl Acad Sci U S A 111, E426-34 - Ruprecht JJ, King MS, Zögg T, Aleksandrova AA, Pardon E, Crichton PG, Steyaert J & Kunji ERS (2019)
The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier.
Cell 176, 435-447.e15