ELECTRON MICROSCOPY OF MITOCHONDRIAL TRANSPORT PROTEINS
In early 2003, we obtained the projection structure of the yeast mitochondrial ADP/ATP carrier Aac3p, representing the first published structural information of a mitochondrial carrier.
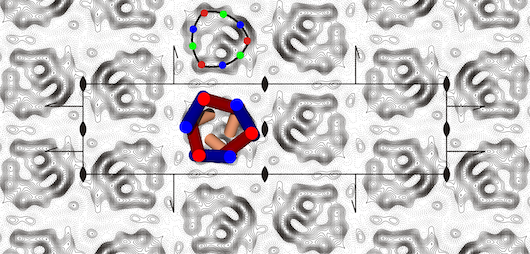
Figure Projection map at 5 Å resolution of the yeast ADP/ATP carrier with the specific inhibitor atractyloside bound.
The projection structure was solved by electron crystallography to 5 Å [1] and showed that the protein was a monomer, not an integrated dimer, as had been postulated [2,3]. The three-fold pseudo-symmetrical arrangement of the density peaks were in agreement with the three homologous sequence repeats, typical of mitochondrial carriers. The density distribution was consistent with the carrier being a bundle of six transmembrane α-helices with a central pore for the translocation of adenine nucleotides. Based on hydropathy profiling, conservation analysis and secondary structure predictions, a new topology model was proposed, in which the signature motif PX[DE]XX[RK] was present at the end of transmembrane α-helices H1, H3 and H5 [1]. It was also predicted that the matrix loops would contain short α-helices, but they could not be localised in the projection map. There was an unexplained asymmetric density peak in the central part of the carrier, which subsequently turned out to be an α-helical extension of the first transmembrane α-helix [4]. We are interested in using electron microscopy to solve the structure of mitochondrial transport proteins.
REFERENCES
Projection structure of the atractyloside-inhibited mitochondrial ADP/ATP carrier of Saccharomyces cerevisiae.
J Biol Chem 278, 36985-36988
Mitochondrial carriers function as monomers.
Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism.
Proc Natl Acad Sci U S A 111, E426-E434