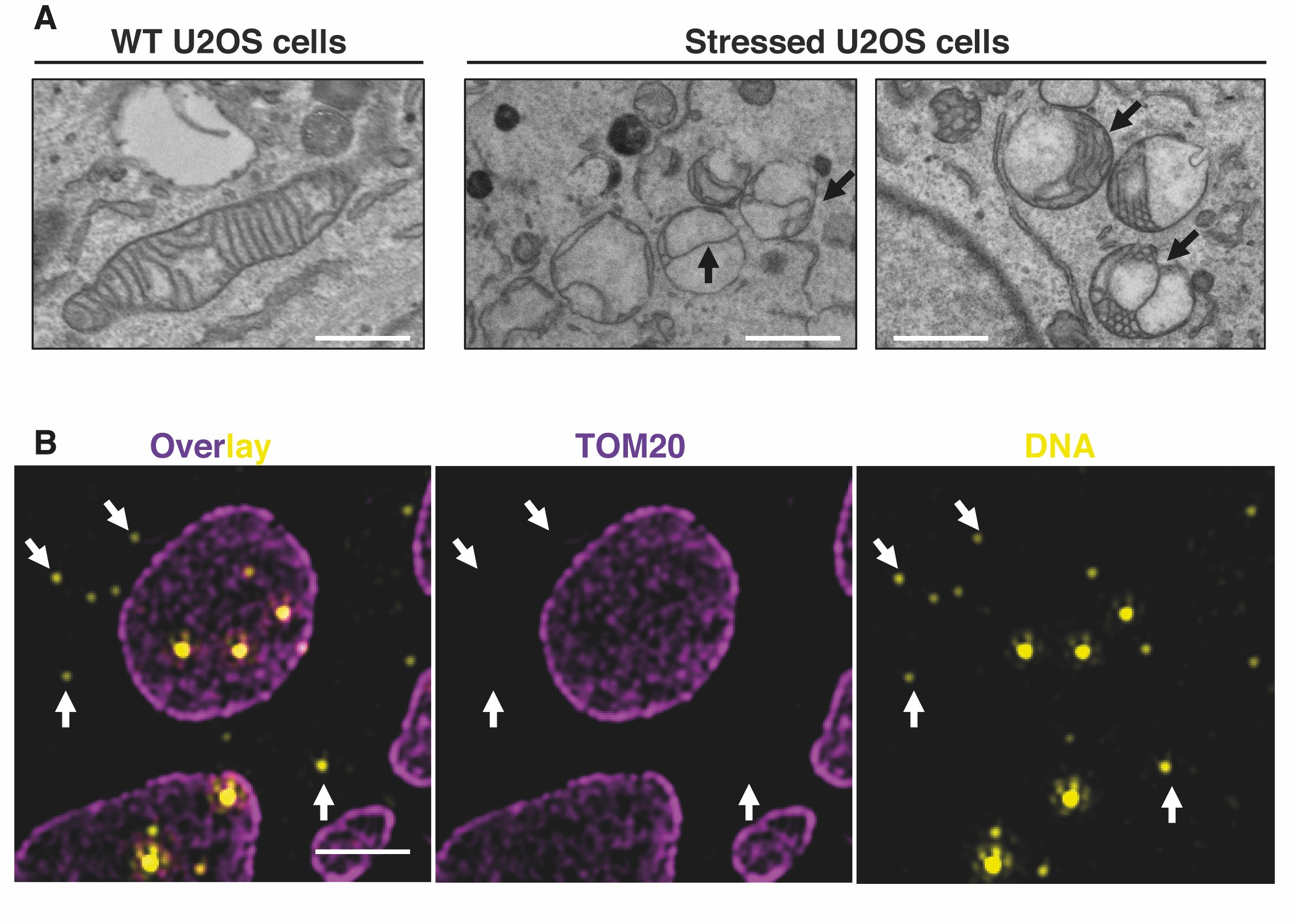
Figure 3. (A) Representative transmission electron microscopy (TEM) images showing inner mitochondrial membrane (IMM) structures defects in U20S-stressed cells compared to untreated cells. Black arrows indicate IMM remodelling defects. Scale bars: 500 nm. (B) Representative N-Structured Illumination Microscopy (N-SIM) super-resolution image showing enlarged mitochondria and cytosolic mtDNA upon mitochondrial stress. Mitochondria and DNA were labelled using anti-TOM20 and anti-DNA antibodies, respectively. Scale bar: 2 um.
The mitochondrial genome consists of a single circular molecule that encodes 22 tRNAs and 13 protein subunits required for oxidative phosphorylation (OXPHOS) activity and adenosine triphosphate (ATP) production. Each mitochondrion contains multiple mtDNA copies that are bound to packaging proteins forming structures known as nucleoids. mtDNA copy number refers to the number of mitochondrial genomes per cell and is indicative of mitochondrial health [1]. It has been established that both mitochondrial fission and fusion processes are essential to ensure a proper mtDNA content along the mitochondrial network [2],[3],[4],[5]. Therefore, an adequate ratio of mitochondrial division and fusion events is essential to ensure mtDNA distribution, to avoid deleterious effects on mtDNA maintenance and to prevent the progression of diseases. However, the precise mechanisms by how mitochondrial morphology regulates mtDNA dynamics are not well understood.
In addition, recent discoveries have shown that not only nuclear or pathogen DNA can drive antiviral signaling and cellular inflammation, but also mitochondrial mtDNA. Similarly, to immunogenic bacterial DNA, mtDNA released in the cytosol is able to trigger different inflammatory responses. These signalling pathways were initially described in the cell death context, but recent studies have highlighted that other mitochondrial stresses including bacterial/viral infection, oxidative stress or mtDNA instability, were also able to induce mtDNA release in the cytosol triggering inflammation [6],[7].
We aim to elucidate how mitochondrial membrane remodelling (Figure 3A) regulates both mtDNA quality dynamics and the release of mtDNA in the cytosol (Figure 3B). To this purpose, we employ multiple in cellulo and in vivo models coupled to high-resolution microscopy methods to characterise new metabolic pathways and proteins involved in mtDNA dynamics and release, to elucidate how mitochondrial membrane dynamics control these phenomena and how these events are linked to cellular homeostasis.
REFERENCES
- Stewart, J. B. & Chinnery, P. F. (2021)
Extreme heterogeneity of human mitochondrial DNA from organelles to populations
Nat Rev Genet 22, 106-118. doi:10.1038/s41576-020-00284-x - Ishihara, T. et al. (2015)
Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development
Mol Cell Biol 35, 211-223. doi:10.1128/MCB.01054-14 - Chen, H. et al. (2010)
Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations
Cell 141, 280-289. doi:10.1016/j.cell.2010.02.026 - Tezze, C. et al. (2017)
Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence
Cell Metab 25, 1374-1389 e1376. doi:10.1016/j.cmet.2017.04.021 - Lieber, T., Jeedigunta, S. P., Palozzi, J. M., Lehmann, R. & Hurd, T. R. (2019)
Mitochondrial fragmentation drives selective removal of deleterious mtDNA in the germline
Nature 570, 380-384. doi:10.1038/s41586-019-1213-4 - West, A. P. & Shadel, G. S. (2017)
Mitochondrial DNA in innate immune responses and inflammatory pathology
Nat Rev Immunol 17, 363-375. doi:10.1038/nri.2017.21 - Riley, J. S. & Tait, S. W. (2020)
Mitochondrial DNA in inflammation and immunity
EMBO Rep 21, e49799. doi:10.15252/embr.201949799