THIOL PROTEOMICS AND ACYLATION
Altering the redox state of cysteine residues on protein surfaces is an important response to environmental challenges. We have investigated modifications to protein thiols in enriched mitochondrial fractions using a modified form of 2D electrophoresis that we have called Redox-DIGE. We have observed a number of cysteine residues that are sensitive enough to respond to plausible in vivo concentrations (low µM) of reactive oxygen or nitrogen species [1] [2].
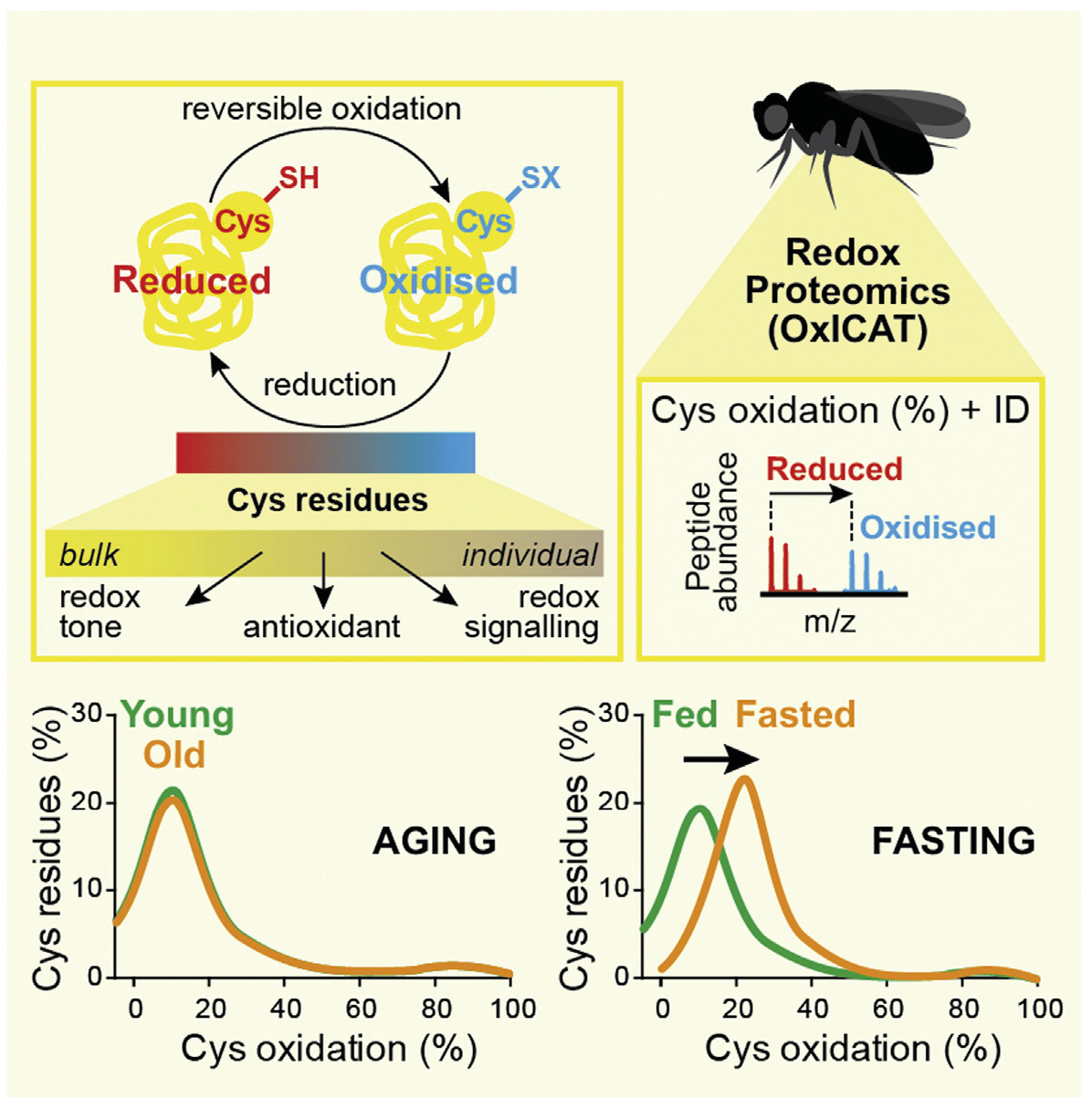
We have used a redox proteomic technique called oxidative isotope-coded affinity tags (OxICAT) to assess cysteine-residue redox changes. We used this approach to simultaneously identify and quantify the redox state of several hundred cysteine residues in Drosophila melanogaster in vivo during ageing and fasting [3]. We have also used it to show that in mouse in vivo protein S-nitrosation occurs in response to the combination of ischemia and nitrite [4].
Current work in the lab has focused on non-enzymatic reversible cysteine S-acetylation/S-acylation by acetyl-CoA and how this impacts on irreversible lysine N-acetylation/N-acylation. Surface cysteine residues readily react with acyl-CoAs and once the acyl moiety is localised on the surface of the protein it can be transferred to nearby lysine residues in a reaction that is enhanced by proximity [5][6]. These reactive sites on the surfaces of protein are less conserved within the genomes of vertebrates [6]. Current work in the lab is focused on why this low stoichiometry (~0.1%) acylation is detrimental.
REFERENCES
- (2007)
Detection of reactive oxygen species-sensitive thiol proteins by redox difference gel electrophoresis: implications for mitochondrial redox signaling.
J Biol Chem 282, 22040-51 - (2010)
Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation.
Biochem J 430, 49-59 - (2015)
Fasting, but Not Aging, Dramatically Alters the Redox Status of Cysteine Residues on Proteins in Drosophila melanogaster.
Cell Rep 11, 1856-65 - (2017)
Identification and Quantification of Protein S-nitrosation by Nitrite in the Mouse Heart during Ischemia.
J Biol Chem 35, 1486-14495 - (2017)
Non-enzymatic N-acetylation of Lysine Residues by AcetylCoA Often Occurs via a Proximal S-acetylated Thiol Intermediate Sensitive to Glyoxalase II.
Cell Rep 18, 2105-2112 - (2018)
Proximal Cysteines that Enhance Lysine N-Acetylation of Cytosolic Proteins in Mice Are Less Conserved in Longer-Living Species.
Cell Rep 24, 1445-1455