Mitochondrial retrograde signalling
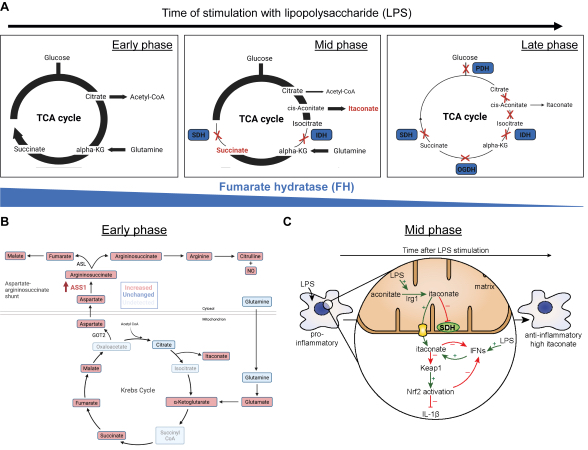
Figure: TCA cycle remodelling in inflammatory macrophages
There is a growing body of evidence that now sheds light on how mitochondria communicate with the cytosol to initiate key biological events, promote homeostasis, and counteract stress conditions, including in immunity and inflammation. Mitochondria are known to perform several major roles within the eukaryotic cell, the production of ATP via oxidative phosphorylation (OXPHOS), regulating iron–sulfur cluster biogenesis and cellular iron metabolism, the support of biosynthetic pathways, and the orchestration of cell death [1]. As such, mitochondria are essential for several biological processes in eukaryotic organisms, namely cellular proliferation, differentiation, and stress adaptation. While, classically, it is thought that the nucleus is responsible for dictating and regulating mitochondrial function, rarely was it thought that mitochondria can in turn generate signals that influence nuclear function. However, as functioning mitochondria are required for such important cellular processes, feedback systems must exist to prevent a metabolic crisis and cell death. The existence of signalling mechanisms between mitochondria and the nucleus raises many important fundamental questions and suggests a fourth major role of the mitochondria is to operate as signalling organelles that influence gene expression and other cellular effector functions. The tricarboxylic acid cycle (TCA) cycle, also known as the Krebs cycle and citric acid cycle (CAC), is an amphibolic pathway operating in mitochondria that has emerged as a central metabolic hub in most eukaryotic organisms. Importantly, the TCA cycle is the gateway to the aerobic metabolism of any fuel molecule, such as carbohydrates, lipids or amino acids that can be transformed into acetyl-CoA or an intermediate of the TCA cycle itself.
The TCA cycle undergoes dynamic remodelling following inflammatory activation (Fig. 1A-C) and this is important for the regulation of macrophage effector functions [2,3]. This remodelling is notably defined by the induction of an inflammatory aspartate-argininosuccinate shunt connecting the TCA cycle to nitric oxide (NO) production (Fig. 1B) and an increase in the production of the cis-aconitate-derived metabolite itaconate (Fig. 1C). Itaconate can subsequently inhibit succinate dehydrogenase (SDH), alkylate protein cysteine residues on key immune regulatory targets and act directly as an anti-bactericidal agent [4]. In addition, we have shown that suppression of the TCA cycle enzyme Fumarate Hydratase (FH) leads to impaired mitochondrial bioenergetics and increases levels of fumarate [5]. This suppression then regulates stress-responsive transcription factor activation and cytokine production via fumarate-mediated protein succination or drives the release of immunostimulatory mitochondrial RNA to regulate the Type I IFN response [5]. Understanding the connection between metabolism and signalling may reveal new insights into inflammatory and autoimmune disease processes, as well as identifying novel anti-inflammatory metabolic compounds and targets.
The Ryan group is particularly interested in understanding the contribution of mitochondrial metabolite signalling and nucleic acid release to infection and inflammatory diseases.
References
- Ryan DG#, Frezza C, O’Neill LAJ. (2020)
TCA cycle signalling and the evolution of eukaryotes.
Curr Opin Biotechnol 68, 72-88. doi:10.1016/j.copbio.2020.09.014
#Corresponding - Ryan DG, O’Neill LAJ. (2020)
Krebs cycle reborn in macrophage immunometabolism.
Annu Rev Immunol 38, 289-313. doi:10.1146/annurev-immunol-081619-104850 - Ryan DG, Murphy M, Frezza C et al. (2018)
Coupling Krebs cycle metabolites to signalling in immunity and cancer.
Nat Metab 1, 16-33. doi:10.1038/s42255-018-0014-7 - 4. Mills E*, Ryan DG*, Prag H et al. (2018)
Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1.
Nature 556, 113-117. doi:10.1038/nature25986
*Joint-first - Hooftman A*#, Peace C*, Ryan DG*# et al. (2023)
Macrophage fumarate hydratase restrains mtRNA-mediated interferon production.
Nature 556, 113-117. doi:10.1038/s41586-023-05720-6
*Joint-first #Co-corresponding