MITOCHONDRIAL CALCIUM IN HEALTH AND NEURODEGENERATION
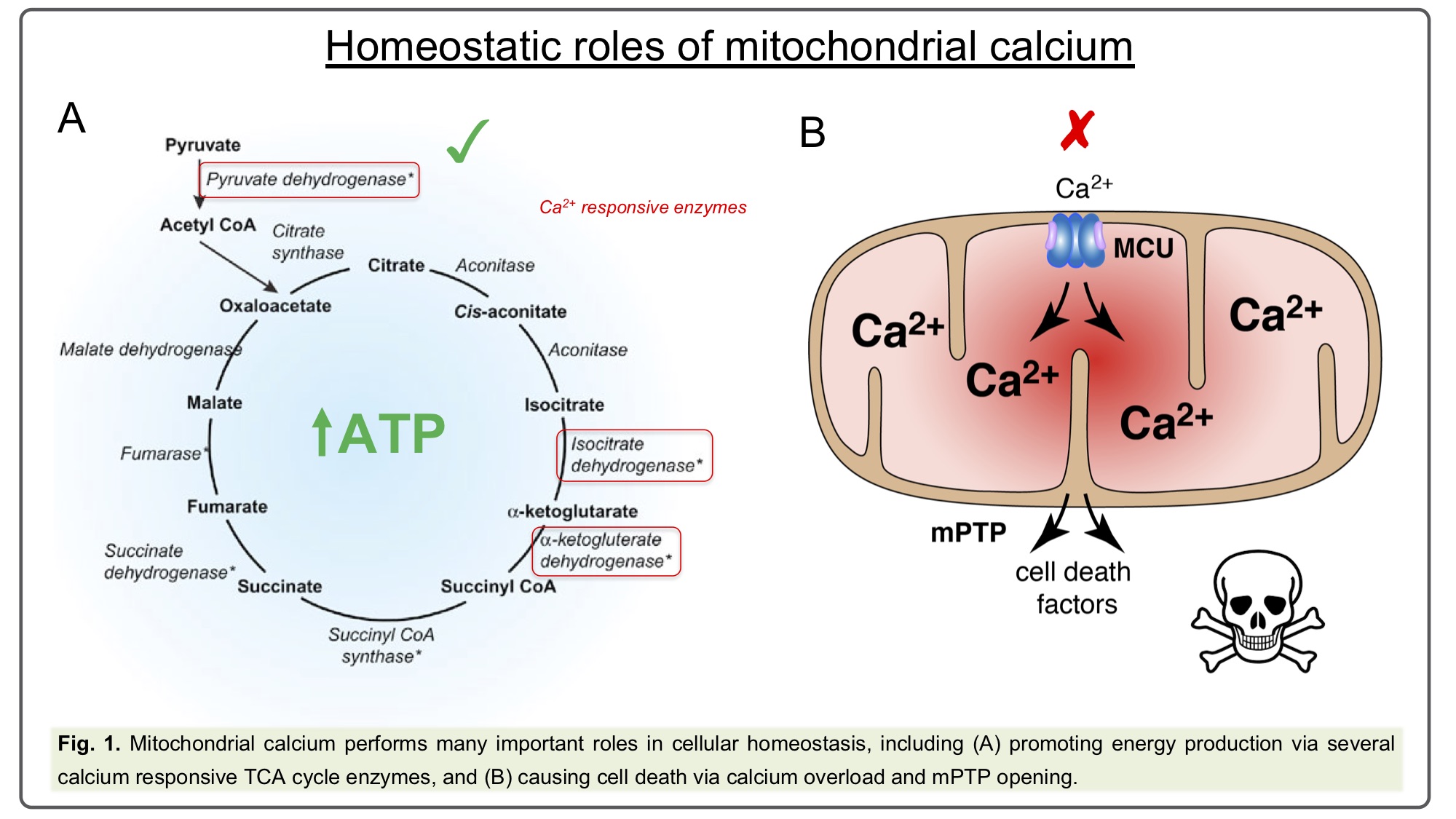
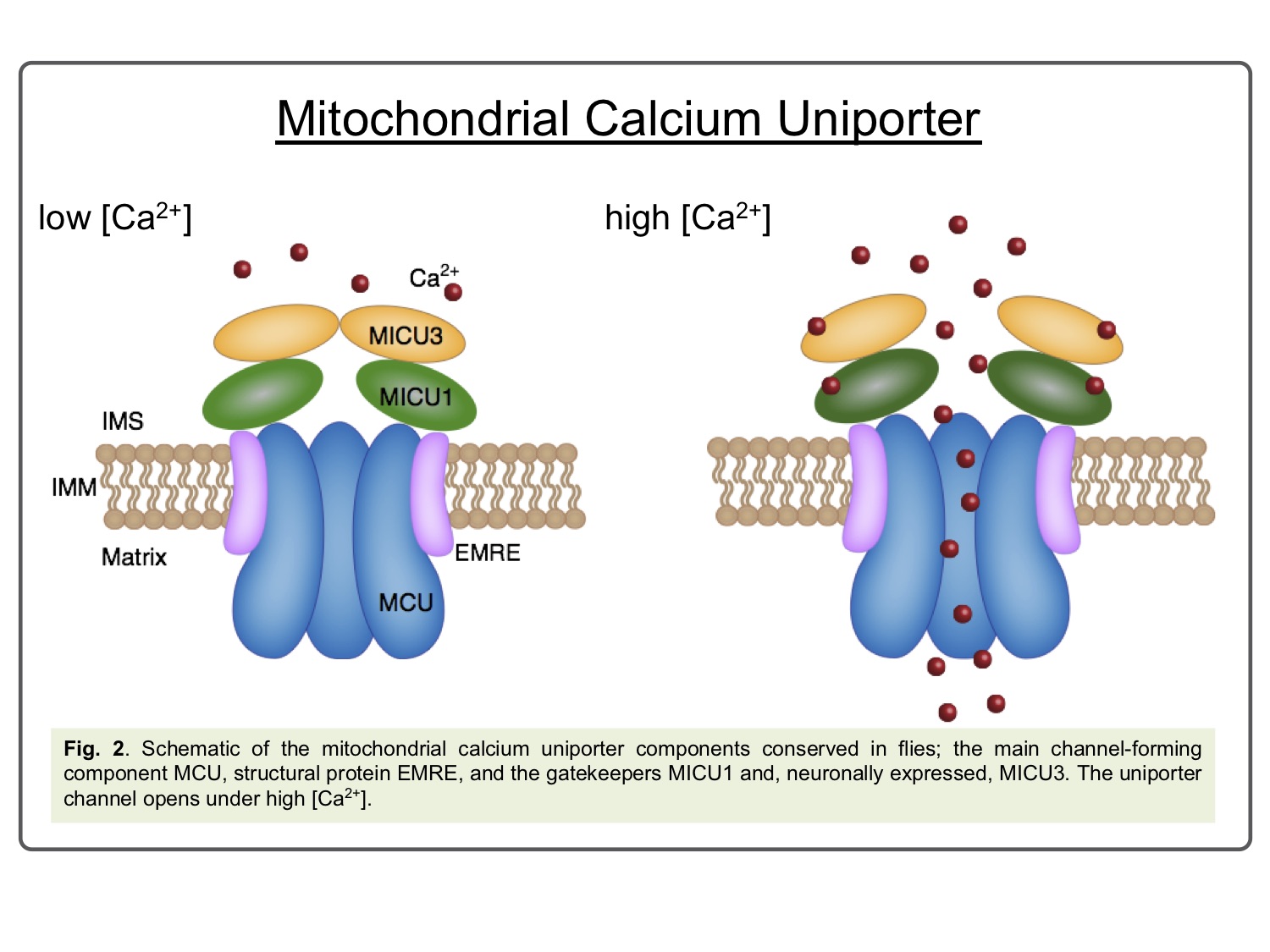
Mitochondrial calcium [Ca2+]m has long been established to play a key role in a wide array of cellular homeostatic processes as diverse as bioenergetics and cell death (Fig. 1), and has been linked to disease conditions as varied as cancer, ischemia-reperfusion injury and neurodegeneration[1],[2],[3]. [Ca2+]m uptake is mediated by the highly selective Mitochondrial Calcium Uniporter complex, while the Na2+/Ca2+ exchanger, NCLX, provides the predominant route for calcium extrusion. The core uniporter components are: MCU as the main pore-forming protein, a small structural component, EMRE, and the regulator subunits, MICU1-3 (Fig. 2). All these components are generally highly conserved across eukaryotes, including most metazoans and plants, reflecting their ancient and fundamental role[4].
The composition and function of the uniporter has been well-characterised n vitro and in cell culture models and the physiological role of uniporter is beginning to emerge with in vivo characterisation of knockout mutants[5]. However, current data present a complex and sometimes contradictory picture. We have generated a comprehensive genetic toolkit to analyse the core uniporter components that are conserved in Drosophila – MCU, EMRE, MICU1 and MICU3 (Drosophila lack MICU2)[6]. Our initial characterisation of these uniporter components has revealed some surprising outcomes. Flies lacking the essential channel components MCU or EMRE are completely viable, with only mild phenotypes. In contrast, MICU1 KO causes developmental lethality, consistent with loss of its gatekeeping activity leading to unregulated Ca2+ uptake. Most surprisingly, this was not rescued by loss of MCU or EMRE, suggesting a uniporter-independent role of MICU1. These reagents will be an important resource for investigating the diversity of uniporter function in health and disease.
With this toolkit we will interrogate the functional roles of the core uniporter components in vivo, and apply them to a variety of disease conditions. For instance, we will aim to better understand the fundamentals of [Ca2+]m homeostasis and how dysregulated [Ca2+]m contributes to disease.
REFERENCES
- Granatiero V, De Stefani D & Rizzuto R (2017)
Mitochondrial calcium handling in physiology and disease.
Adv Exp Med Biol 982, 25-47 - Mammucari C, Raffaello A, Reane DVecellio & Rizzuto R (2016)
Molecular structure and pathophysiological roles of the mitochondrial calcium uniporter.
Biochim Biophys Acta 1863, 2457-2464 - Liao Y, Dong Y & Cheng J (2017)
The function of the mitochondrial calcium uniporter in neurodegenerative disorders.
Int J Mol Sci 18, 248 - Bick AG, Calvo SE & Mootha VK (2012)
Evolutionary diversity of the mitochondrial calcium uniporter.
Science 336, 886 - Liu JC, Parks RJ, Liu J, Stares J, Rovira II, Murphy E & Finkel T (2017)
The in vivo biology of the mitochondrial calcium uniporter.
Adv Exp Med Biol 982, 49-63 - Tufi R, Gleeson TP, von Stockum S, Hewitt VL, Lee JJ, Terriente-Felix A, Sanchez-Martinez A, Ziviani E & Whitworth AJ (2019)
Comprehensive genetic characterization of mitochondrial Ca2+ uniporter components reveals their different physiological requirements in vivo.
Cell Rep 27, 1541-1550.e1545