MECHANISMS TO HANDLE AND MITIGATE MTDNA MUTATIONS
The mitochondrial genome (mtDNA) in animals encodes 13 subunits of the respiratory chain and ATP synthase, along with the tRNAs and rRNAs necessary for their production[1]. MtDNA mutations can give rise to devastating mitochondrial diseases, and have been implicated as a driving force in the ageing process[2], with high levels of mtDNA mutations being found in various neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases[3].
The consequences of heteroplasmic mtDNA mutations has been studied in vivo using the proofreading (exonuclease) deficient variant of the mtDNA polymerase, POLG. The mtDNA ‘mutator’ mouse exhibit many characteristics of a premature ageing syndrome and shortened lifespan[4],[5]. However, the mutational heterogeneity that arises in this model has led to considerable debate about the pathogenic entity and whether point mutations are actually driving ageing.
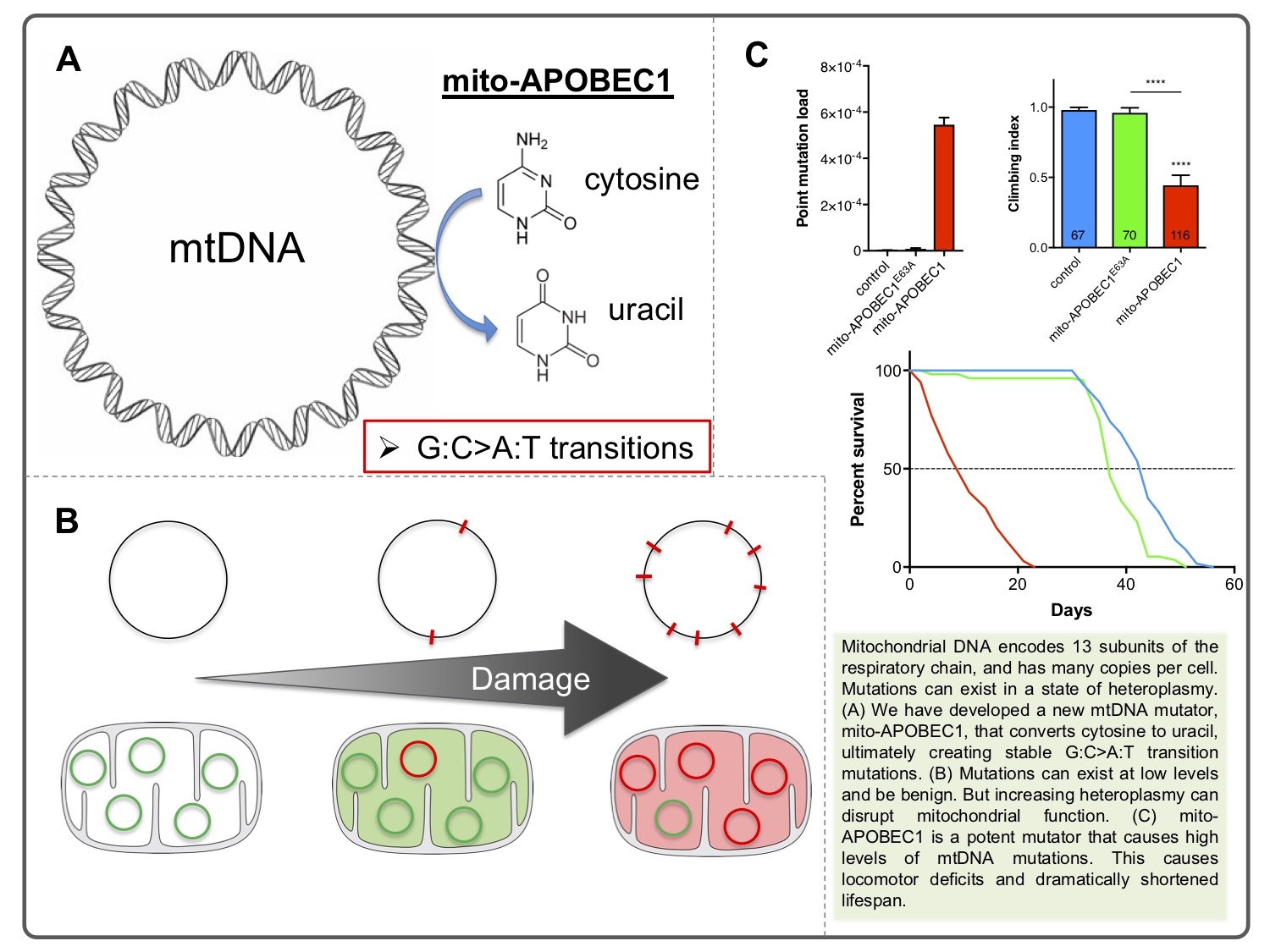
We have recently developed an alternative mtDNA mutator model system by targeting the cytidine deaminase APOBEC1 to mitochondria. APOBEC1 catalyses the deamination of cytosine to uracil (C>U), which can cause a mutagenic transition of a C:G to T:A point mutation that mirrors the predominant mutation profile in human ageing[6]. The “mito-APOBEC1” flies generate high level of somatic mtDNA mutations which severely limit mitochondrial function, organismal vitality and lifespan[7]. We will use this model to investigate the impact of mtDNA mutations on cellular homeostatic processes and the mechanisms which mitigate the accumulation of mtDNA mutations such as mitophagy and other quality control measures.
A summary of this work has been published in the Atlas of Science.
REFERENCES
- Chinnery PFrancis & Hudson G (2013)
Mitochondrial genetics.
Br Med Bull 106, 135-159 - Larsson N-G (2010)
Somatic mitochondrial DNA mutations in mammalian aging.
Annu Rev Biochem 79, 683-706 - Keogh MJ & Chinnery PF (2015)
Mitochondrial DNA mutations in neurodegeneration.
Biochim Biophys Acta 1847, 1401-1411 - Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C & Prolla TA (2005)
Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging.
Science 309, 481-484 - Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT & Larsson N-G (2004)
Premature ageing in mice expressing defective mitochondrial DNA polymerase.
Nature 429, 417-423 - Kennedy SR, Salk JJ, Schmitt MW & Loeb LA (2013)
Ultra-sensitive sequencing reveals an age-related increase in somatic mitochondrial mutations that are inconsistent with oxidative damage.
PLoS Genet 9, e1003794 - Andreazza S, Samstag CL, Sanchez-Martinez A, Fernandez-Vizarra E, Gomez-Duran A, Lee JJ, Tufi R, Hipp MJ, Schmidt EK, Nicholls TJ, Gammage PA, Chinnery PF, Minczuk M, Pallanck LJ, Kennedy SR & Whitworth AJ (2019)
Mitochondrially-targeted APOBEC1 is a potent mtDNA mutator affecting mitochondrial function and organismal fitness in Drosophila.
Nat Commun 10, 3280