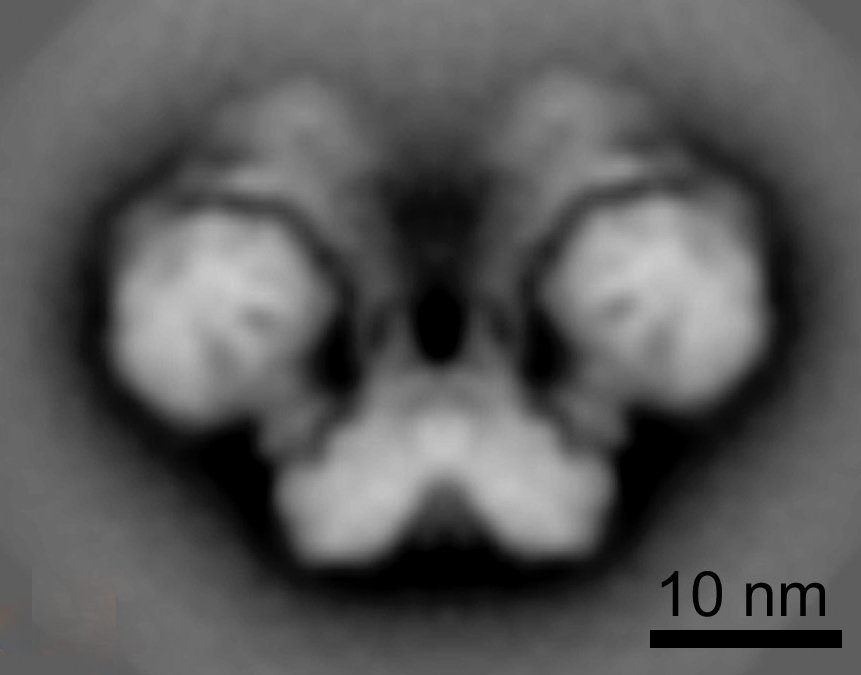
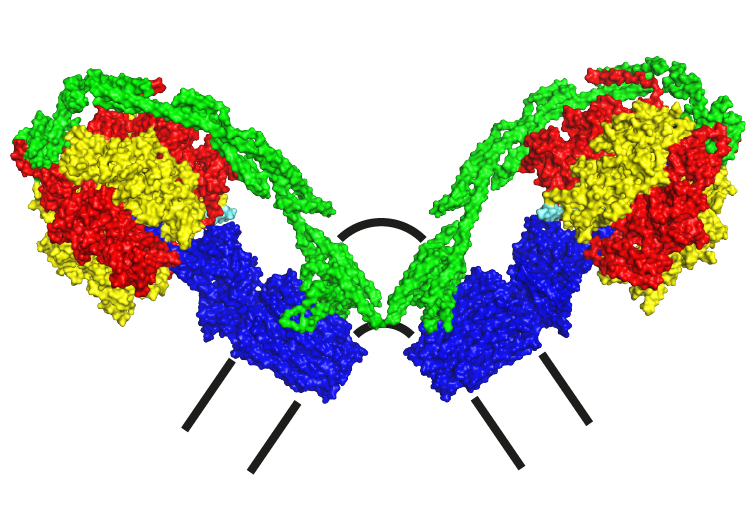
DIMERS
In mitochondria, ATP synthase complexes are arranged in rows of dimers along the edges of the cristae [1] [2] [3] [4] [5]. Our aim is to define the structural roles of subunits e, f, g, DAPIT, 6.8PL and ATP8 in the dimerisation of monomeric complexes and in the linking of dimers together into long rows on the cristae edges. [6] [7] [8] [9] [5]. It has been proposed that the mitochondrial permeability transition pore is associated with dimers of ATP synthase [10], but we can find no evidence to support this proposition (see here).
REFERENCES
- Paumard P, Vaillier J, Coulary B, Schaeffer J, Soubannier V, Mueller DM, Brèthes D, di Rago J-P & Velours J (2002)
The ATP synthase is involved in generating mitochondrial cristae morphology.
EMBO J 21, 221-230 - Dudkina NV, Heinemeyer J, Keegstra W, Boekema EJ & Braun H-P (2005)
Structure of dimeric ATP synthase from mitochondria: an angular association of monomers induces the strong curvature of the inner membrane.
FEBS Lett 579, 5769-5772 - Strauss M, Hofhaus G, Schröder RR & Kühlbrandt W (2008)
Dimer ribbons of ATP synthase shape the inner mitochondrial membrane.
EMBO J 27, 1154-60 - Davies KM, Strauss M, Daum B, Kief JH, Osiewacz HD, Rycovska A, Zickermann V & Kühlbrandt W (2011)
Macromolecular organization of ATP synthase and complex I in whole mitochondria.
Proc Natl Acad Sci U S A 108, 14121-14126 - Hahn A, Parey K, Bublitz M, Mills DJ, Zickermann V, Vonck J, Kühlbrandt W & Meier T (2016)
Structure of a complete ATP synthase dimer reveals the molecular basis ofiInner mitochondrial membrane morphology.
Mol Cell 63, 445-456 - Arnold I, Pfeiffer K, Neupert W, Stuart RA & Schägger H (1998)
Yeast mitochondrial F1F0-ATP synthase exists as a dimer: identification of three dimer-specific subunits.
EMBO J 17, 7170-7178 - Arselin G, Giraud M-F, Dautant A, Vaillier J, Brèthes D, Coulary-Salin B, Schaeffer J & Velours J (2003)
The GxxxG motif of the transmembrane domain of subunit e is involved in the dimerization/oligomerization of the yeast ATP synthase complex in the mitochondrial membrane.
Eur J Biochem 270, 1875-1884 - Bustos DM & Velours J (2005)
The modification of the conserved GXXXG motif of the membrane-spanning segment of subunit g destabilizes the supramolecular species of yeast ATP synthase.
J Biol Chem 280, 29004-29010 - Paumard P, Arselin G, Vaillier J, Chaignepain S, Bathany K, Schmitter JMarie, Brèthes D & Velours J (2002)
Two ATP synthases can be linked through subunits i in the inner mitochondrial membrane of Saccharomyces cerevisiae.
Biochemistry 41, 10390-10396 - Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G & Bernardi P (2013)
Dimers of mitochondrial ATP synthase form the permeability transition pore.
Proc Natl Acad Sci U S A 110, 5887-5892