CALCIUM REGULATION OF MITOCHONDRIAL CARRIERS
There are two mitochondrial carriers that are activated when cytosolic calcium levels are high and inactivated when cytosolic calcium levels are low. The mitochondrial aspartate/glutamate carrier imports glutamate in symport with a proton and exports aspartate for the malate-aspartate shuttle, urea cycle, gluconeogenesis and myelin synthesis. The mitochondrial ATP-Mg/Pi carrier imports adenine nucleotides from the cytosol and exports phosphate to control the size of the adenine nucleotide pool in the mitochondrial matrix in response to cellular energetic demands.
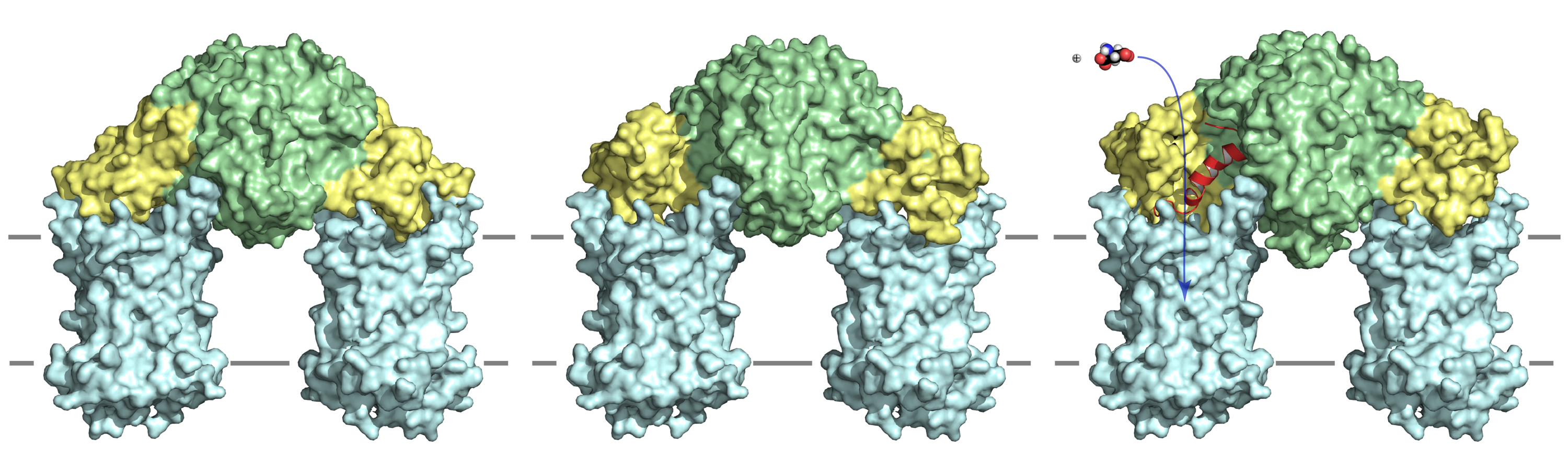
Figure Calcium regulation of the mitochondrial aspartate/glutamate carrier; the apo-state (left), the calcium-bound state without the C-terminal helix (middle), and the calcium-bound state with the C-terminal helix bound to the hydrophobic cleft.
The mitochondrial aspartate/glutamate carriers have a unique three-domain structure, comprising a calcium-regulated N-terminal domain with eight EF-hands, a mitochondrial carrier domain, and a C-terminal domain [1]. We have solved the atomic structure of the N- and C-terminal domain in the calcium-bound and apo-state, elucidating the mechanism of calcium regulation [2]. The eight EF-hands of N-terminal domain form a unique structure. EF-hands 4-8 form a static unit (green) and are involved in dimerization of the carrier, which is unique as other mitochondrial carriers are monomeric [3] [4] [5] [6] [7] EF-hands 1-3 form the calcium-responsive mobile unit (yellow), which changes conformation upon the binding of calcium to EF-hand 2. Upon calcium binding, the amphipathic helix of the C-terminal domain (red helix) binds to the N-terminal domain, opening a vestibule for substrates to enter the carrier domains (light blue). In the absence of calcium, the mobile unit closes the vestibule and prevents the substrate from entering the carrier, blocking transport. These structures have provided a structural model for the regulation of the mitochondrial aspartate/glutamate carriers by calcium. The movie below shows the conformational changes that occur when the regulatory domain changes from the calcium-bound state to the apo-state, and vice versa. EF-hands 1-8 of the N-terminal domain are shown in a rainbow colour scheme, whereas the amphipathic helix of the C-terminal domain is shown in wheat.
Movie Transition between the calcium-bound and calcium-free state of the regulatory domain of the mitochondrial aspartate/glutamate carrier. If the movie does not show, please refresh the web page.
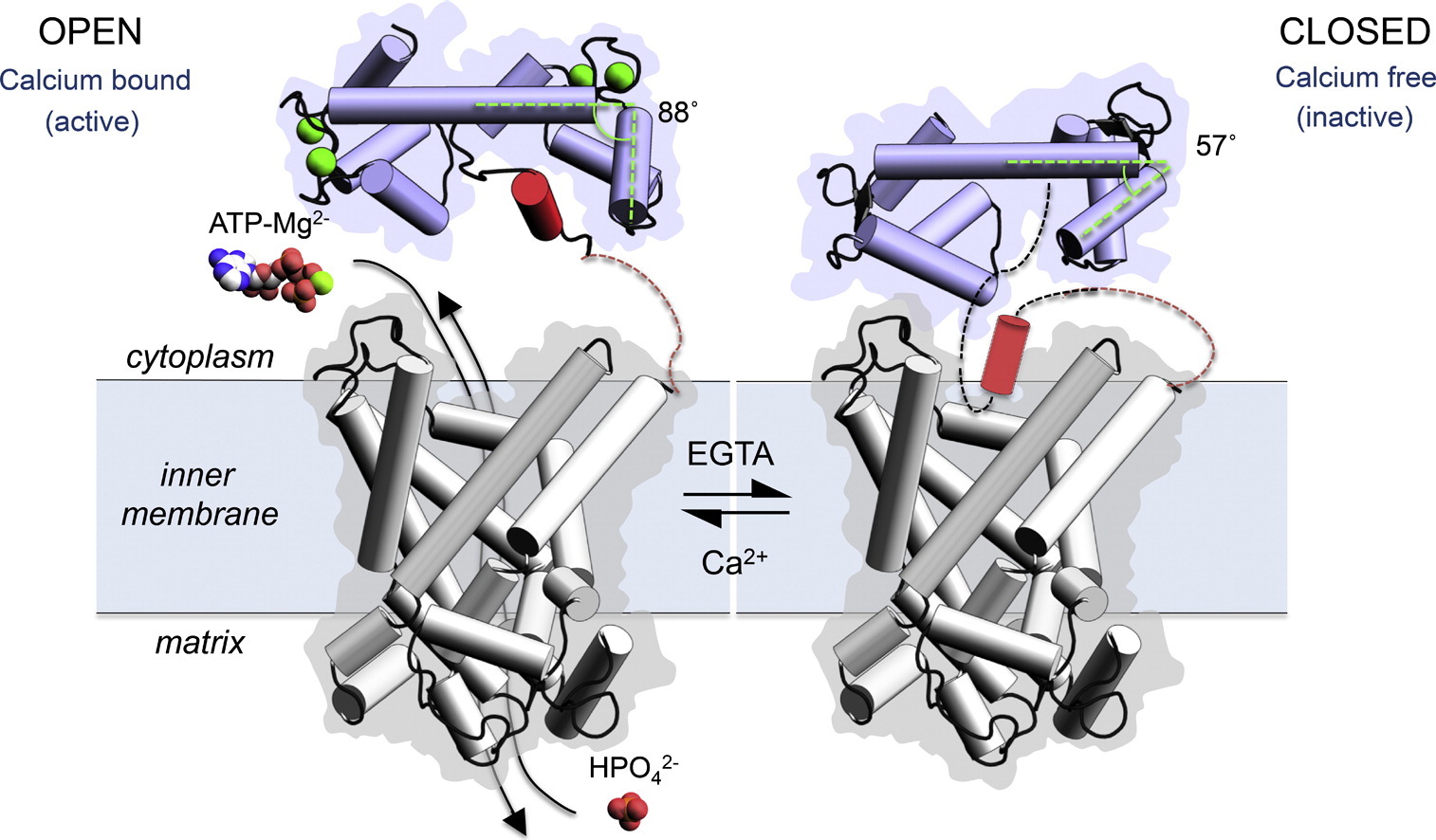
The mitochondrial ATP-Mg/Pi carrier [8] also consists of three domains; (i) the N-terminal regulatory domain, which is formed of two pairs of fused calcium-binding EF-hands, (ii) the C-terminal mitochondrial carrier domain, which is involved in transport, and (iii) a linker region with an amphipathic α-helix. The structure of the regulatory domain shows that the amphipathic α-helix is bound to the regulatory domain in a hydrophobic cleft of EF-hand 3/4. Upon release of calcium, EF-hands close, leading to the release of the amphipathic α-helix from the regulatory domain. We have proposed a mechanism for ATP-Mg/Pi carriers in which the amphipathic α-helix becomes mobile upon release of calcium and blocks the transport of substrates across the mitochondrial inner membrane by the carrier domain [9].
Figure The proposed regulatory mechanism between calcium-bound state of HsAPC-1 where the carrier is active and the calcium-free state of HsAPC-1 where the carrier is inactive.
Movie Transition between the calcium-bound and calcium-free state of the regulatory domain of the mitochondrial ATP-Mg/phosphate carrier. If the movie does not show, please refresh the web page.
REFERENCES
- (2001)
Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria.
EMBO J 20, 5060-9 - (2014)
Calcium-induced conformational changes of the regulatory domain of human mitochondrial aspartate/glutamate carriers.
Nat Commun 5, 5491 - (2007)
Yeast mitochondrial ADP/ATP carriers are monomeric in detergents as demonstrated by differential affinity purification.
J Mol Biol 371, 388-95 - (2007)
The yeast mitochondrial ADP/ATP carrier functions as a monomer in mitochondrial membranes.
Proc Natl Acad Sci U S A 104, 10830-4 - (2006)
Yeast mitochondrial ADP/ATP carriers are monomeric in detergents.
Proc Natl Acad Sci U S A 103, 16224-9 - (2010)
Mitochondrial carriers function as monomers.
Biochim Biophys Acta 1797, 817-31 - (2015)
Uncoupling protein 1 binds one nucleotide per monomer and is stabilized by tightly bound cardiolipin.
Proc Natl Acad Sci U S A 112, 6973-8 - (2004)
Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution.
J Biol Chem 279, 30722-30 - (2015)
Calcium-induced conformational changes in the regulatory domain of the human mitochondrial ATP-Mg/Pi carrier.
Biochim Biophys Acta 1847, 1245-53