TRANSPORT MECHANISM OF MITCHONDRIAL CARRIERS
In 2008, we proposed a new transport mechanism for mitochondrial carriers based on the disruption and formation of the two salt bridge networks on either side of the substrate binding site. This alternating access mechanism involves the simultaneous rotation of three domains around the central substrate translocation pathway, which is a unique mechanism for transport proteins. The structural mechanism of transport will explain the molecular basis of the diseases associated with dysfunctional mitochondrial carriers.
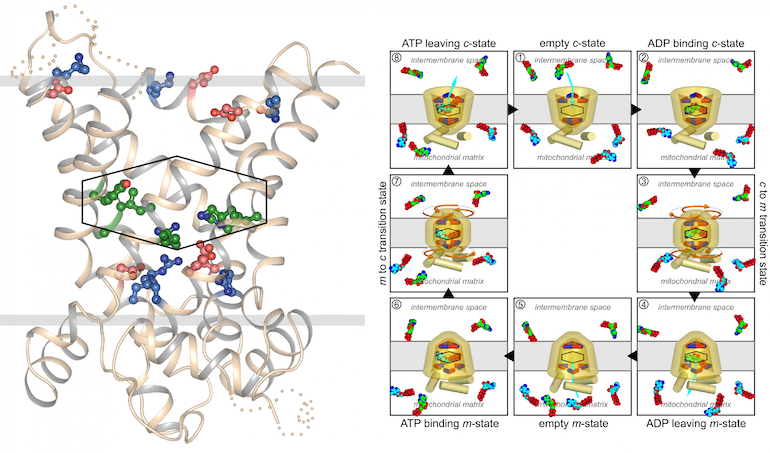
Figure Mitochondrial carriers cycle between a matrix and cytoplasmic state by formation and disruption of the cytoplasmic salt bridge network (top) and matrix salt bridge network (bottom).
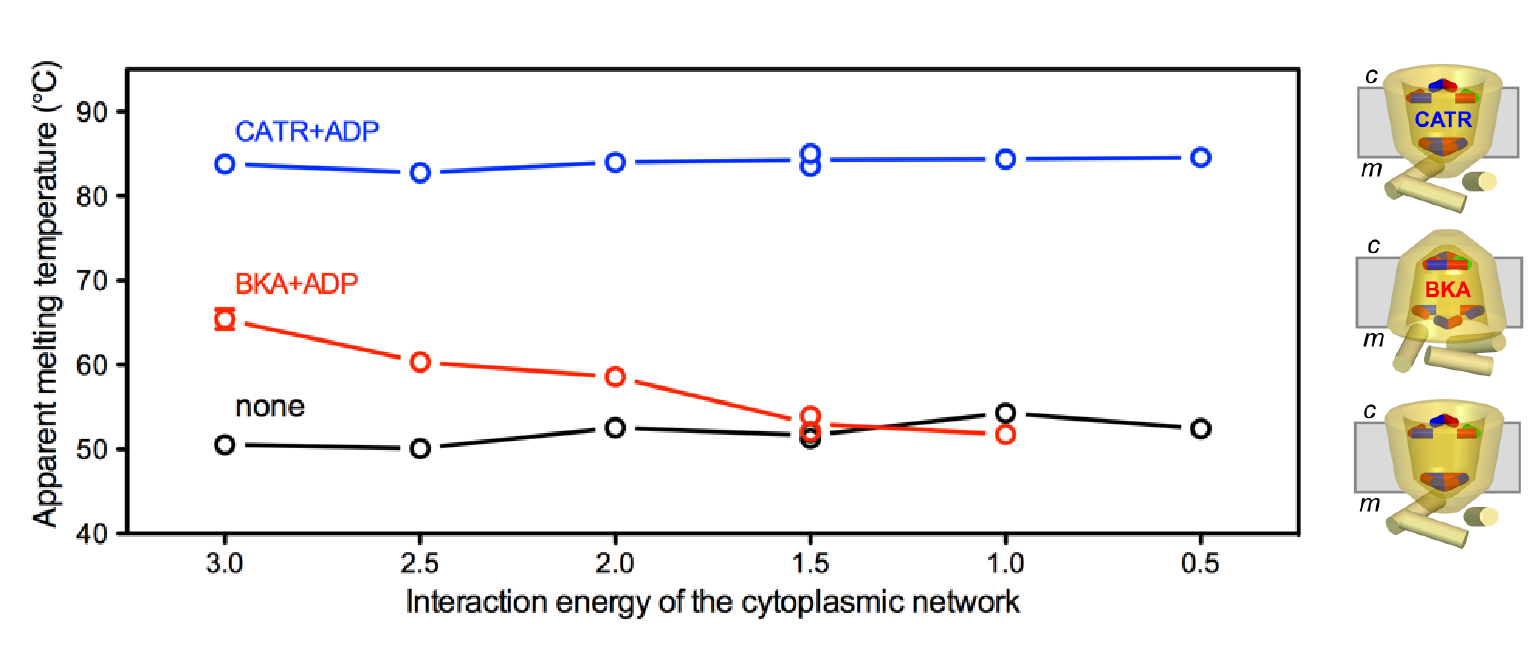
Mitochondrial carriers function as monomers [1] [2] [3] [4], meaning that the previously proposed mechanisms based on the dimeric structure were no longer tenable [5] [6] [7]. Mitochondrial carriers have a well conserved three-fold pseudo-symmetry in their sequences and structures [1] [8], which must be important for the structural mechanism of transport [9]. Highly conserved symmetrical residues were found to flank the central substrate binding site at the water-membrane interfaces. One set consists of the three PX[DE]XX[RK] signature motifs, which form a salt bridge network on the matrix side of the cavity, when the substrate binding site is open to the mitochondrial intermembrane space [8]. The second set has three symmetrical [FY][DE]XX[RK] motifs on the cytoplasmic side of the cavity, which forms a salt bridge network when the substrate binding site is accessible from the mitochondrial matrix [9]. The opening and closing of the carrier to the cytoplasmic and matrix side of the membrane is coupled to the disruption and formation of the two salt bridge networks. Recently, we have provided experimental evidence showing that the postively and negatively charged residues of the cytoplasmic network interact in the transport cycle [4] and that the cytoplasmic salt bridge network forms in the matrix state by using thermostability studies of carriers locked in the matrix or cytoplasmic state by inhibitors [10]. The structures of the mitochondrial ADP/ATP carrier in the cytoplasmic state and the matrix state have shown that this structural mechanism indeed holds [4] [11] [12].
We want to resolve the molecular basis of mitochondrial diseases associated with mitochondrial carriers by determining the structural mechanism of transport.
Figure The thermostability of the bongkrekic acid-inhibited (BKA) matrix state is dependent on the interaction energy of the cytoplasmic salt bridge network, whereas the carboxyatractyloside-inhibited (CATR) cytoplasmic state is not [10]. ADP was added to obtain a homogeneous population trapped in a single state.
REFERENCES
- Kunji ERS & Harding M (2003)
Projection structure of the atractyloside-inhibited mitochondrial ADP/ATP carrier of Saccharomyces cerevisiae.
J Biol Chem 278, 36985-8 - Bamber L, Harding M, Monné M, Slotboom D-J & Kunji ERS (2007)
The yeast mitochondrial ADP/ATP carrier functions as a monomer in mitochondrial membranes.
Proc Natl Acad Sci U S A 104, 10830-4 - Bamber L, Harding M, P Butler JG & Kunji ERS (2006)
Yeast mitochondrial ADP/ATP carriers are monomeric in detergents.
Proc Natl Acad Sci U S A 103, 16224-9 - Ruprecht JJ, Hellawell AM, Harding M, Crichton PG, McCoy AJ & Kunji ERS (2014)
Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism.
Proc Natl Acad Sci U S A 111, E426-34 - Kunji ERS & Crichton PG (2010)
Mitochondrial carriers function as monomers.
Biochim Biophys Acta 1797, 817-31 - Kunji ERS (2012)
Structural and mechanistic aspects of mitochondrial transport proteins.
In Comprehensive Biophysics, Edward H Egelman, ed. (Elsevier) pp. 174 - 205 - Kunji ERS & Robinson AJ (2010)
Coupling of proton and substrate translocation in the transport cycle of mitochondrial carriers.
Curr Opin Struct Biol 20, 440-7 - Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJ-M & Brandolin G (2003)
Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside.
Nature 426, 39-44 - Robinson AJ, Overy C & Kunji ERS (2008)
The mechanism of transport by mitochondrial carriers based on analysis of symmetry.
Proc Natl Acad Sci U S A 105, 17766-71 - King MS, Kerr M, Crichton PG, Springett R & Kunji ERS (2016)
Formation of a cytoplasmic salt bridge network in the matrix state is a fundamental step in the transport mechanism of the mitochondrial ADP/ATP carrier.
Biochim Biophys Acta 1857, 14-22 - Ruprecht JJ, King MS, Zögg T, Aleksandrova AA, Pardon E, Crichton PG, Steyaert J & Kunji ERS (2019)
The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier.
Cell 176, 435-447.e15 - Ruprecht JJ & Kunji ERS (2019)
Structural changes in the transport cycle of the mitochondrial ADP/ATP carrier.
Curr Opin Struct Biol 57, 135-144