Submitted by Penny Peck on Tue, 28/06/2022 - 17:57
The results of a research study carried out by members of the MBU's group on Mitochondrial Transport Processes, published in Nature Communications, has identified the substrate binding mechanism of the ADP/ATP carrier, also called adenine nucleotide translocase.
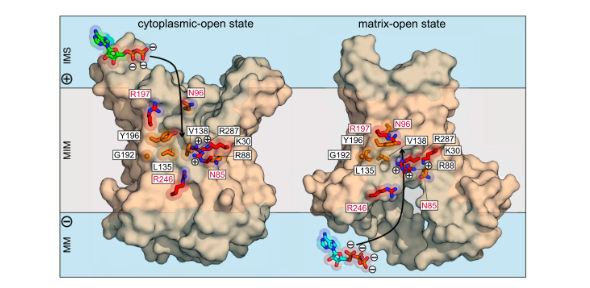
These transport proteins have unique features that enable them to achieve a high flux of ADP and ATP across the mitochondrial inner membrane to sustain our daily activities. Furthermore, they have evolved to navigate the challenge of importing negatively charged ADP against the large membrane potential generated across the mitochondrial inner membrane by the respiratory chain. Despite being studied for more than 55 years, there are still many open questions about the transport mechanism, in particular about substrate binding and its effect on the interconversion between the matrix- and cytoplasmic-open states.
Structures of the inhibited cytoplasmic- and matrix-open states have confirmed an alternating access transport mechanism, but the molecular details of substrate binding remain unresolved. In this research article, the authors evaluate the role of the solvent-exposed residues of the translocation pathway in the process of substrate binding. They identify the main binding site, comprising three positively charged and a set of aliphatic and aromatic residues, which bind ADP and ATP in both states. Additionally, there are two pairs of asparagine/arginine residues on opposite sides of this site that are involved in substrate binding in a state-dependent manner. Thus, the substrates are directed through a series of binding poses, inducing the conformational changes of the carrier that lead to their translocation. The properties of this site also explain the electrogenic and reversible nature of adenine nucleotide transport.
In conclusion, the properties of the identified binding site explain many features of the mechanism that heretofore had no molecular explanation. The same principles, as discovered here, could apply to the binding and transport of substrates for other members of the SLC25 mitochondrial carrier family, which are currently under investigation.
Publication reference:

