Submitted by Penny Peck on Mon, 20/06/2022 - 12:18
The membrane contact sites between the endoplasmic reticulum (ER) and the mitochondria, called mitochondria-ER contact sites (MERCs), are dynamic signalling platforms essential for cellular viability. Indeed, MERCs are not only required to maintain mitochondrial membrane integrity by regulating the biogenesis and trafficking of different phospholipids but also to control critical processes such as calcium (Ca2+) transport. Disruption of MERCs usually leads to mitochondrial dysfunction and compromises ER function, causing a variety of pathologies including neurological diseases. For example, it is well known that impaired Ca2+ transport at MERCs affects synaptic transmission and plasticity. Therefore, deregulation in phospholipid trafficking at this specific interface can cause ER-shaping defects and induce altered lipid homeostasis in axons, which could represent a pathogenic mechanism in motor neuron degenerative diseases (MNDs), such as hereditary spastic paraplegias (HSPs).
A new international and collaborative study published in Brain from the labs of Dr. Julien Prudent (MRC MBU, University of Cambridge), Prof. Andrew Crosby and Dr. Emma Baple (University of Exeter) identified a new gene, TMEM63C, in which they identified mutations associated with a degenerative disease that affects the upper motor neuron cells in the nervous system leading to HSP. The Exeter team used cutting-edge genetic sequencing techniques to investigate the genome of three families with individuals affected by HSP and identified mutations in the TMEM63C gene as the cause of the disease, which manifests in the patients with multiple symptoms including muscle stiffness, weakness and wasting. In addition, using both biochemical and cutting-edge microscopy approaches, Dr Luis-Carlos Tabara Rodriguez (a Postdoctoral Fellow in Dr. Prudent’s lab), showed that a subset of TMEM63C localises at the MERCs interface. Importantly, TMEM63C loss led to both ER and mitochondrial morphology defects in multiple cell types, including differentiated motor-neuron like cells. This discovery is in line with increasing evidence linking HSP-associated genes with phospholipid homeostasis mediated at MERCs and highlights the physiological importance of mitochondria-ER communication for cellular homeostasis and viability. Finally, this study opens potential new therapeutic opportunities for the diagnostic and treatment of MNDs through the analysis and manipulation of MERCs.
Publication reference:
Luis Carlos Tábara, Fatema Al-Salmi, Reza Maroofian, Amna Mohammed Al-Futaisi, Fathiya Al-Murshedi, Joanna Kennedy, Jacob O Day, Thomas Courtin, Aisha Al-Khayat, Hamid Galedari, Neda Mazaheri, Margherita Protasoni, Mark Johnson, Joseph S Leslie, Claire G Salter, Lettie E Rawlins, James Fasham, Almundher Al-Maawali, Nikol Voutsina, Perrine Charles, Laura Harrold, Boris Keren, Edmund R S Kunji, Barbara Vona, Gholamreza Jelodar, Alireza Sedaghat, Gholamreza Shariati, Henry Houlden, Andrew H Crosby, Julien Prudent, Emma L Baple, TMEM63C mutations cause mitochondrial morphology defects and underlie hereditary spastic paraplegia, Brain, 2022;, awac123, https://doi.org/10.1093/brain/awac123
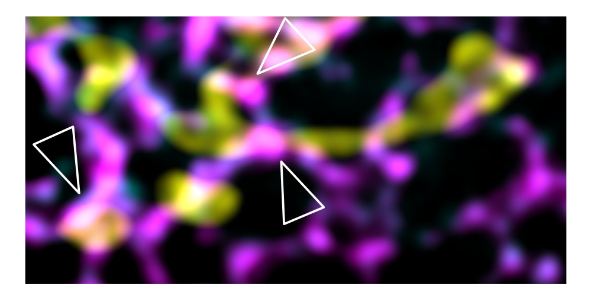
Figure legend: Representative N-SIM super resolution microscopy image of HeLa cells expressing TMEM63C-Flag, showing TMEM63C-Flag foci accumulation at MERCs (white arrows). Flag, mitochondria, and ER were labelled with anti-Flag (magenta), anti-TOM20 (yellow) and anti-Calnexin (cyan) antibodies, respectively. Scale bars: 2 µm.

