Submitted by Penny Peck on Thu, 25/02/2021 - 16:01
In a research article published in Proceedings of the National Academy of Sciences, USA, Tobias Spikes, Martin Montgomery and John Walker have shown how the mobility in the interfaces between monomers in dimeric bovine ATP synthase participates in forming the characteristic and ever-changing ultrastructure of inner mitochondrial membranes. This article has been selected by the PNAS as a research highlight.
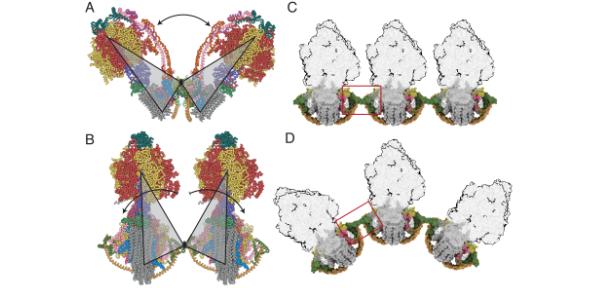
Pairs of rotary ATP synthases complexes embedded in the inner membranes of mitochondria dimerize in their membrane domains, and the dimers form long rows on cristae tips, helping to endow them with their characteristic and mobile tubular shape. The structural analyses of bovine dimers show that the monomers pivot and translate at wedge-shaped structures in their membrane domains. The structures suggest how dimeric ATP synthases can fashion themselves to the wide range of oligomeric arrangements that are observed in mitochondria, allowing at the same time the ATP synthase to produce ATP under a wide range of physiological conditions.
The present article follows on from an earlier recent one by the same authors, also published in the PNAS, that established the atomic structures of the dimeric ATP synthases, showing how the mechanical rotation required for making ATP is generated by applying a voltage tangentially to the rotors.
References:

