Submitted by Penny Peck on Mon, 03/08/2020 - 00:00
These exciting results of research carried out in the MBU’s Mitochondrial Complex I Group, led by Professor Judy Hirst, have been published in Nature Structural & Molecular Biology.
Daniel Grba, PhD student and first author, gives this overview of the key findings:
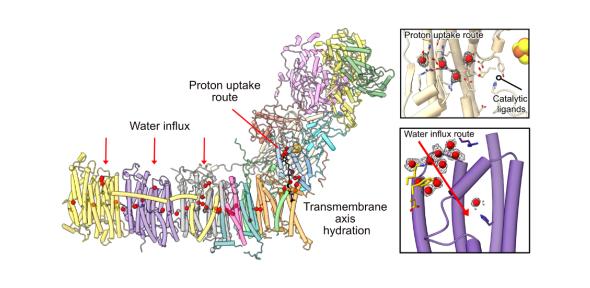
Using single-particle cryo-electron microscopy (cryo-EM) we have obtained a structure of complex I (NADH:ubiquinone oxidoreductase) from the yeast model organism Yarrowia lipolytica resolved to 2.7 Å, the highest published resolution for this enzyme. Mitochondrial complex I powers ATP synthesis by oxidative phosphorylation, exploiting the energy from ubiquinone reduction by NADH to drive protons across the energy-transducing inner membrane. Its central role in metabolism and cellular energy production makes its dysfunction a cause for neuromuscular and degenerative diseases and metabolic disorders. These include diseases linked directly to complex I mutations, such as Leber’s hereditary optic neuropathy, as well as those associated with its dysfunction such as Parkinson’s disease. The mechanism of how proton pumping is coupled to substrate catalysis is still unknown. Our high-resolution data allows water molecules in vital functional regions of the complex to be observed, including potential water influx points into the proton-pumping elements, key proton-translocating residues that span the centre of the ~150 Å-long transmembrane arm, and a potential proton uptake route at the substrate reduction site.
Using our new information, we reassess other recent cryo-EM analyses of eukaryotic complex I to better understand movement of secondary structure elements between these structures and their role in hydration of the transmembrane subunits. We also deconvolute structural changes governing the global transition the mammalian enzyme undergoes when it adopts a protective state known as ‘deactive’, that occurs during ischemia–reperfusion injury, and are able to deduce how the ubiquinone substrate binding site is connected to the transmembrane subunits involved in signalling the lateral proton-translocating relay.
Observing water molecules is a vital step forward in understanding the function and dysfunction of complex I, moving towards understanding mechanisms behind the coupling of substrate catalysis to proton pumping, previously only predicted with simulations. The resolution revolution continues to power forward in cryo-EM structural biology and it will be an essential tool in fully understanding this enigmatic respiratory machine.
Grba, D.N., Hirst, J. Mitochondrial complex I structure reveals ordered water molecules for catalysis and proton translocation. Nat Struct Mol Biol (2020). https://doi.org/10.1038/s41594-020-0473-x


