Submitted by Penny Peck on Thu, 06/03/2025 - 12:19
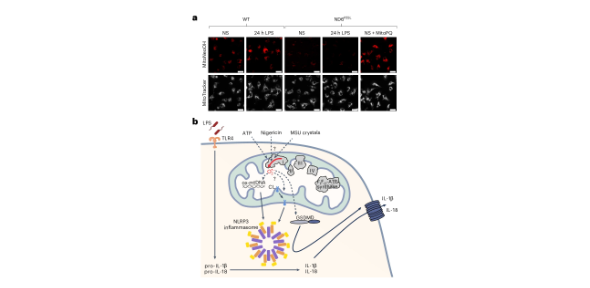
Pro-inflammatory macrophages produce mitochondria-derived reactive oxygen species (mtROS) such as superoxide, but it is not understood how it is produced or how it affects macrophage effector functions. Published in Nature Metabolism, results of a study led by Professor Mike Murphy, who that mitochondrial superoxide is produced by reverse electron transport at complex I of the respiratory chain and regulates pro-inflammatory cytokine production.
It is now clear that mitochondria are far more than the powerhouses of the cell; they are also information-processing hubs that regulate cell function and fate through multiple signalling pathways. This is particularly evident in the context of immune cells, in which mitochondria-derived signals regulate immune cell function.. One such signal in pro-inflammatory macrophages is thought to be elevated mitochondria-derived reactive oxygen species (mtROS). However, neither the mechanism or site of generation, nor the importance of mtROS as a regulator of macrophage effector functions were known. This is in part due to the difficulty of measuring mtROS and in particular in pinpointing the site of mtROS production within the electron transport chain. In this study, the researchers set out to address these outstanding questions to better understand how mtROS regulates immune cell function.
The study shows how mitochondrial superoxide production arises in pro-inflammatory macrophages and then regulates IL-1β release following NLRP3 inflammasome activation. More broadly, this work illustrates the role of mtROS as a signal that regulates cell function. The tools, genetic models and approaches used here will be useful in studying mtROS signalling in many other contexts.
Although this study shows that mtROS is a regulator of NLRP3 inflammasome activation, the precise pathway by which RET-produced ROS regulates IL-1β release is still not clear. IL-1β is released through gasdermin D pores following NLRP3 inflammasome activation and these findings support recent studies that suggest ROS-mediated oxidation of gasdermin D affects its oligomerisation and pore formation. However, other signals such as fragmented and modified mitochondrial DNA or oxidised cardiolipin may also be involved in this pathway.
Further work will focus on determining the mechanistic details by which RET-produced ROS regulates IL-1β release, such as how the mitochondrial signals are passed to the cytoplasm and whether these pathways offer novel therapeutic opportunities.
Publication reference
Casey, A.M., Ryan, D.G., Prag, H.A. et al. Pro-inflammatory macrophages produce mitochondria-derived superoxide by reverse electron transport at complex I that regulates IL-1β release during NLRP3 inflammasome activation. Nat Metab (2025). https://doi.org/10.1038/s42255-025-01224-x

