The function of TLCD proteins
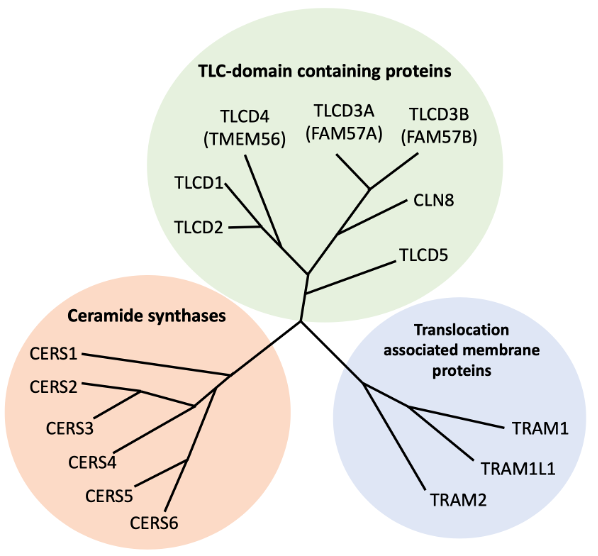
Figure: Simplified depiction of human TLCD protein phylogeny
The Tram-Lag-CLN8 (TLC) domain is a conserved multi-pass transmembrane protein domain found in plants, yeast, and animals. In humans, 16 genes encode TLC-domain-containing proteins. Six of these genes encode ceramide synthase enzymes, which catalyse the formation of ceramides, structural lipids in cellular membranes. Another three genes encode TRAM proteins, which facilitate the translocation of nascent protein chains into or through the endoplasmic reticulum membrane. The remaining six genes form a distinct phylogenetic branch, and their functions remain less well understood.
Our laboratory recently discovered that two of these lesser-known genes encode acyltransferases that regulate cellular phospholipid composition. Specifically, we found that TLCD1 is an evolutionarily conserved lysophosphatidylethanolamine acyltransferase that modulates the acyl chain composition of phosphatidylethanolamine, a key mitochondrial phospholipid. Additionally, we discovered that CLN8 is an acyltransferase involved in lysosomal phospholipid bis(monoacylglycero)phosphate synthesis. Our current research focuses on uncovering the enzymatic functions of the remaining TLCD proteins while also gaining deeper insight into how the activity and substrate specificity of all TLCD proteins, including TLCD1 and CLN8, are defined at the molecular level.
Related publication
Sheokand PK, James AM, Jenkins B, K Lysyganicz P, Lacabanne D, King MS, Kunji ERS, Siniossoglou S, Koulman A, Murphy MP & Petkevicius K# (2025)
TRAM-LAG1-CLN8 family proteins are acyltransferases regulating phospholipid composition
Sci Adv 11(8):eadr3723. doi: 10.1126/sciadv.adr3723
#corresponding author
Petkevicius K#, Palmgren H, Glover MS et al. (2022)
TLCD1 and TLCD2 regulate cellular phosphatidylethanolamine composition and promote the progression of non-alcoholic steatohepatitis
Nat Commun 13(1):6020. doi: 10.1038/s41467-022-33735-6
#corresponding author